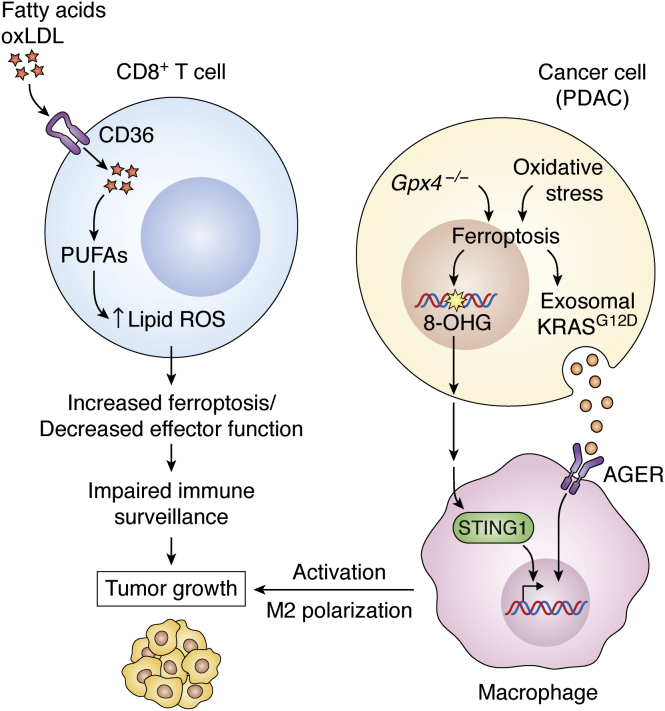
Figure 5.
Protumor roles of ferroptosis in the tumor microenvironment. The accumulation of lipids, including cholesterol, fatty acids, or oxLDLs, in the TME induces the upregulation of CD36 on CD8+ T cells, which leads to further increased uptake of fatty acids and oxLDLs. Elevated fatty acid uptake by CD36 results in higher levels of PUFAs, increased lipid ROS, and enhanced ferroptosis. Ferroptosis of CD8+ T cells decreases effector function, impairs immune surveillance, and promotes tumor growth. Moreover, Gpx4 deletion or high-iron diet promotes pancreatic tumorigenesis via the release of oxidized nucleobases, e.g., 8-OHG, which activates the STING pathway resulting in increased macrophage activation and infiltration and tumor growth. Similarly, oxidative stress in pancreatic cancer cells results in autophagy-induced ferroptosis and the release of KRASG12D packaged in exosomes. Binding of exosomes containing KRASG12D to AGER receptors on macrophages induced polarization of macrophages to “M2” phenotype and increased tumorigenesis. 8-OHG, 8-hyroxyguanosine; AGER, advanced glycosylation end-product specific receptor; CD36, cluster of differentiation 36; Gpx4, glutathione peroxidase 4; oxLDL, oxidized low density lipoprotein; PUFA, polyunsaturated fatty acids; ROS, reactive oxygen species; STING1, stimulator of interferon genes 1.