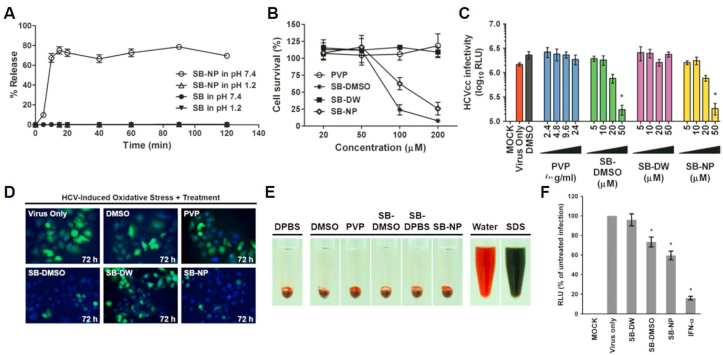
Fig. 8.
A) Dissolution test of silibinin (SB) (filled circle: pH 7.4; filled triangle: pH 1.2) and SB nanoparticles (SB-NPs) (open circle: pH 7.4; open triangle: pH 1.2) by high-performance liquid chromatography analysis. Data shown are mean ± SEM or representative analysis from three independent experiments. B) Cytotoxicity evaluation of SB-NPs on Huh-7 cells by 2,3-bis[2-methoxy-4-nitro-5- sulfophenyl]-2H-tetrazolium-5- carboxyanilide inner salt assay. All data represent mean ± SEM (*p < 0.05) from three independent experiments. C) Determination of antiviral dose–response effect of SB-NPs against reporter cell culture-derived HCV particle (HCVcc) infection (multiplicity of infection (MOI) = 0.01) by luciferase assay. All data represent mean ± SEM (*p < 0.05) from three independent experiments. D) Effect of SB-NPs on HCV-induced oxidative stress in Huh-7 cells by 2’,7′-dichlorofluorescin diacetate (H2DCFDA) staining analysis. E) Haemolytic analysis of SB-NP treatment effect on human erythrocytes. F) SB-NPs inhibit cell culture-derived HCVcc infection of primary human hepatocytes. Primary human hepatocytes inoculated with reporter HCVcc (MOI = 0.01) were treated with clarified insoluble suspension of silibinin in water (SB-DW), silibinin dissolved in dimethyl sulfoxide (SB-DMSO), and SB-NPs and analyzed for luciferase reporter activity following incubation. Interferon (IFN)-α (1000 IU/mL) served as a positive control. Data shown are mean ± SEM (*p < 0.05) from three independent experiments. RLU: relative light units. Adapted with permission [161]. Copyright 2017, BMJ journals.