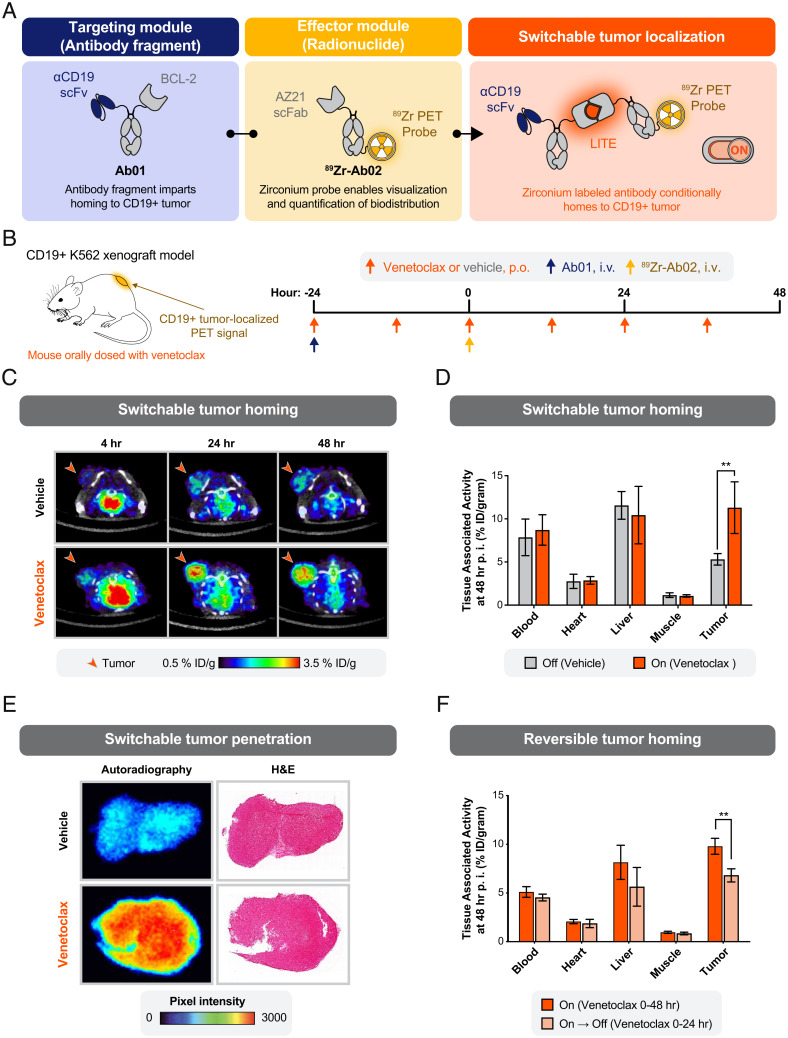
Fig. 3.
Switchable localization of a radionuclide-conjugated antibody to a solid tumor. (A) Schematic of a protein design to enable switchable interaction between a CD19-targeting antibody fragment and an 89Zr-labeled antibody for imaging by PET. (B) Study design to measure the biodistribution of the 89Zr-labeled antibody. Antibodies were injected intravenously (i.v.) into immunocompromised mice bearing tumor xenografts engineered to overexpress human CD19. Venetoclax (10 mg/kg) or vehicle control was delivered by oral gavage (per os [p.o.]). (C) Representative transaxial PET/CT images showed venetoclax-dependent localization of PET signal to the tumor by 4-h postinjection (p.i.) and higher signal at 24 and 48 h. Minimal PET signal was detected in the tumors of mice treated with the oral gavage vehicle. Representative images from a single mouse per condition are shown. (D) Venetoclax treatment resulted in a significant increase in tumor-bound 89Zr-Ab02 as quantified ex vivo (%ID/gram, percent injected dose per gram of tissue). No significant difference in 89Zr-Ab02 uptake was observed between groups among normal tissues (error bars SEM; n = 4 per group; unpaired two-tailed t test; **P = 0.008). (E) Autoradiography and hematoxylin and eosin (H&E) staining of representative tumor sections show the distribution of 89Zr-Ab02 within tumors from mice treated with vehicle or venetoclax. 89Zr-Ab02 penetrates the tumors of mice treated with venetoclax. Data presented are representative of four technical replicates. (F) Tumor binding of 89Zr-Ab02 was significantly reversed when dosing of venetoclax was halted at 24 h after infusion of the antibodies. (error bars SEM; n = 3 to 4 per group; unpaired two-tailed t test; **P = 0.003).