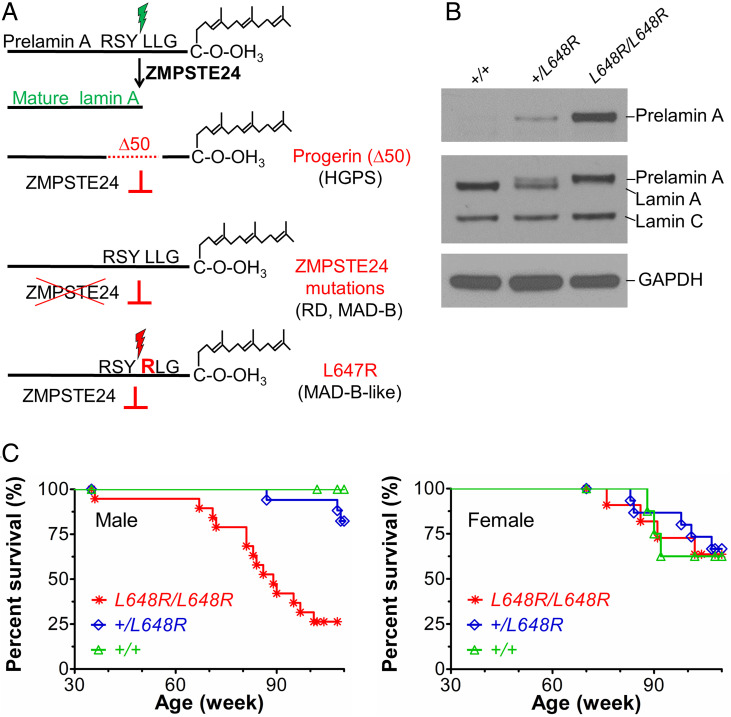
Fig. 1.
Survival of mice with a Lmna L648R mutation corresponding to the human mutation LMNA L647R that encodes an uncleavable variant of prelamin A. (A) Prelamin A is normally processed to lamin A after proteolytic cleavage catalyzed by the zinc metalloprotease ZMPSTE24 between tyrosine (Y) and leucine (L) 647 (648 in mouse), removing a peptide containing the carboxyl-terminal farnesylated cysteine (C). HGPS-causing LMNA mutations generate a prelamin A variant with an internal deletion of 50 amino acids (Δ50) called progerin, which lacks the ZMPSTE24 cleavage site and retains a farnesylated carboxyl-terminal cysteine. ZMPSTE24 loss-of-function mutations cause RD or MAD-B, in which unprocessed farnesylated prelamin A accumulates. LMNA mutation causing a MAD-B-like disorder generates a leucine (L) to arginine (R) substitution at residue 647 of prelamin A blocks ZMPSTE24 processing, leading to expression of a farnesylated variant with only a single amino acid difference. (B) Immunoblots of protein extracts from livers of Lmna+/+ (+/+), Lmna+/L648R (+/L648R), and LmnaL648R/L648R (L648R/L648R) mice. Blots were probed with an antibody specific for prelamin A (Top), an anti-lamin A/C antibody that recognized prelamin A, lamin A, and lamin C (Middle), or anti-GAPDH antibody as loading control (Bottom). (C) Survival curves for male L648R/L648R (n = 19), +/L648R (n = 17), and +/+ (n = 10) mice and female L648R/L648R (n = 11), +/L648R (n = 15), and +/+ (n = 8) mice.