Figure 4.
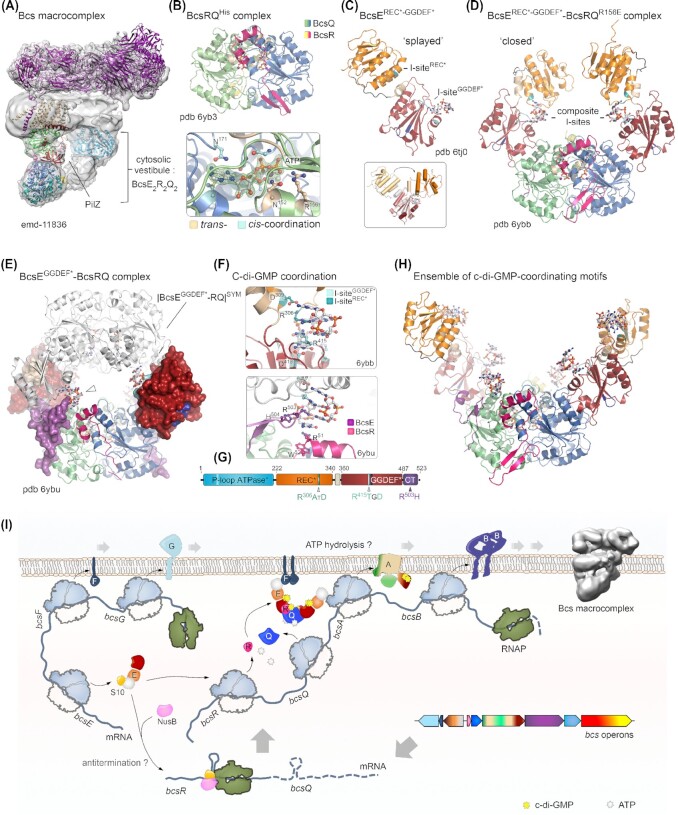
Nucleotide-sensing regulatory components of the E. coli Bcs cellulose secretion macrocomplex. Adapted from (Zouhir et al. 2020; Abidi et al. 2021) (A) Bcs macrocomplex assembly as in Fig. 3 highlighting the central position of c-di-GMP-bound BcsAPilZ within the BcsE2R2Q2 cytosolic vestibule. Densities corresponding to the BcsEREC*-GGDEF* modules are not well resolved, likely due to conformational heterogeneity. (B) Top, crystal structure of a purified BcsRQHis complex. Bottom, zoom-in on one of the two ATP ligands co-purified and co-crystallized with BcsRQHis (pdb 6yb3). Magnesium-coordinating water molecules are also shown as gray spheres. An |Fo|-|Fc| partial electron density map calculated from a model prior to inclusion of the ATP, Mg++ and coordinating water molecules is shown as a green mesh. Trans- and cis-coordinating residues are colored in wheat and cyan, respectively (Abidi et al. 2021). (C) Crystal structure of the isolated BcsBREC*-GGDEF* domain showing a splayed conformation where a single c-di-GMP molecule is bound to the GGDEF* domain's I-site (pdb 6tj0). The two I-site motifs are colored in teal (REC* I-site) and cyan (GGDEF* I-site); the degenerate receiver domain (REC*) is colored in orange; and the degenerate diguanylate cyclase domain (GGDEF*) is colored in deep red. Inset, BcsE conformational changes between the splayed and closed conformations, showing movement of the receiver domain relative to the overlaid GGDEF* module. (D) Crystal structure of the BcsEREC*-GGDEF*–BcsRQR156E complex (pdb 6ybb), featuring a closed BcsEREC*-GGDEF* conformation, in which each domain contributes an I-site RxxD motif to coordinate a c-di-GMP moiety from the intercalated dimeric ligand. (E) Structure and crystal packing of a BcsEGGDEF*–BcsRQ complex (pdb 6byu), with the BcsEGGDEF* domain shown in surface representation and the BcsQ-wrapping C-terminal tail of the protein colored in purple. A symmetry-related BcsEGGDEF*–BcsRQ complex is shown in white and a third c-di-GMP coordinating motif is evident at the BcsEC-tail–BcsR interface. (F) C-di-GMP binding motifs within the crystallized BcsERQ complexes. Top, dimeric c-di-GMP coordination by the composite I-sites with RxxD motif contributions from the REC* and GGDEF* domains in the closed BcsE conformation. The contributions of both sites in c-di-GMP binding have been experimentally confirmed and characterized (Zouhir et al. 2020; Abidi et al. 2021). Bottom, c-di-GMP coordination at the third, crystallographic binding motif shown in panel (E). (G) Domain architecture of full-length BcsE, showing the position and sequence of c-di-GMP binding motifs. (H) A composite structural model of the BcsEREC*-GGDEF*2–BcsR2Q2 complex with c-di-GMP bound at all six coordinating motifs. Although such conformation is unlikely within the assembled Bcs macrocomplex, c-di-GMP-binding motifs in the vestibule are proposed to dynamically contribute to intercalated c-di-GMP retention and recycling for processive synthase activation. (I) Integrated model for E. coli Bcs secretion system assembly: bcsEFG and bcsRQABZC operons are expressed separately as polycistronic mRNAs. BcsE forms a stable equimolar complex with the small ribosomal protein S10, which is also a component of the conserved transcription antitermination machinery (TAC). A second TAC component, NusB, competes with BcsE for S10 binding; however, the physiological role of these BcsE-S10–NusB interactions in vivo remains elusive. Detection of putative intrinsic terminators in the 5′-proximal regions of the bcsRQABZC mRNA has led to an as-yet untested hypothesis of bcsRQABZC expression regulation at the transcription-elongation level (Zouhir et al. 2020). Expressed BcsR and BcsQ stimulate each other's folding and stability to form ATP-bound ‘sandwich’ assemblies, which bind available BcsE and are recruited to the inner membrane via BcsE–BcsF interactions (Zouhir et al. 2020). While ATP hydrolysis is inhibited in the cytosolic BcsERQ complex, it is likely essential at the membrane level, where it could lead to efficient BcsA sorting, assembly and stability within the macrocomplex (Abidi et al. 2021). In addition, the BcsERQ complex directly affects processive glucose polymerization through synthase-proximal c-di-GMP retention and direct structural interaction with the cytosolic BcsA modules (Abidi et al. 2021; Acheson et al. 2021).