Figure 6.
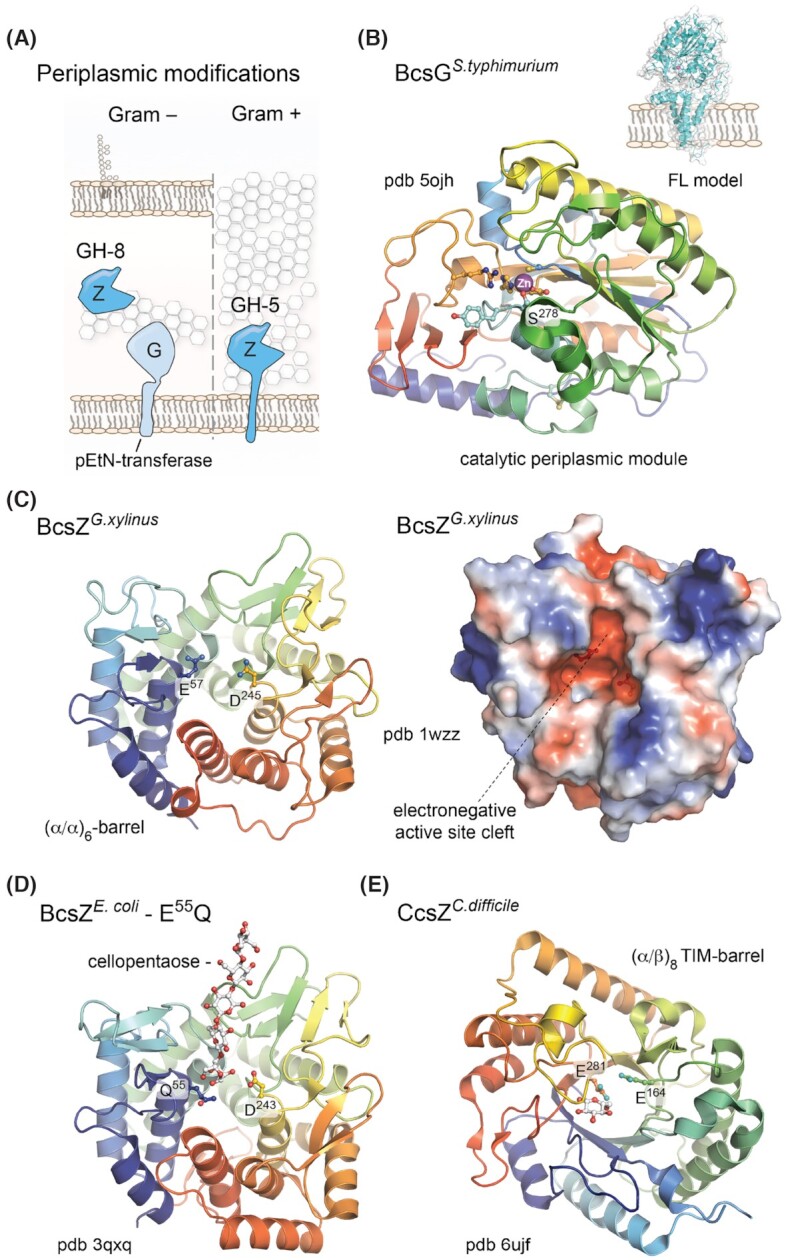
Prevalent periplasmic modifications.(A) Thumbnail representation of the BcsG pEtN-transferase and BcsZ functional homologs in Gram-negative and Gram-positive species. (B) Crystal structure of the BcsG periplasmic domain showing the overall fold of the catalytic module and Zn++ coordination in the active site. Key residues involved in cation coordination and/or essential for the pEtN modification are shown as sticks. Based on Sun et al. (2018) and Anderson et al. (2020). Inset, Robetta-modeled full-length BcsG. (C) Crystal structure of the G. xylinus BcsZ homolog (Yasutake et al. 2006). Left, cartoon representation showing the conserved (α/α)6-barrel fold and the catalytic dyad of acidic (D/E) amino acids as sticks. Right, surface electrostatic potential shown as a red (negative)–blue (positive) gradient. (D) Crystal structure of a cellopentaose-bound catalytically inactive mutant of E. coli BcsZ (Mazur and Zimmer 2011). The oligosaccharide and catalytic dyad are shown as sticks. (E) Crystal structure of the C. difficile CcsZ, showing the (α/β)6 TIM-barrel fold in cartoon and the catalytic dyad as sticks. A single glucose molecule is also seen bound to the protein.
