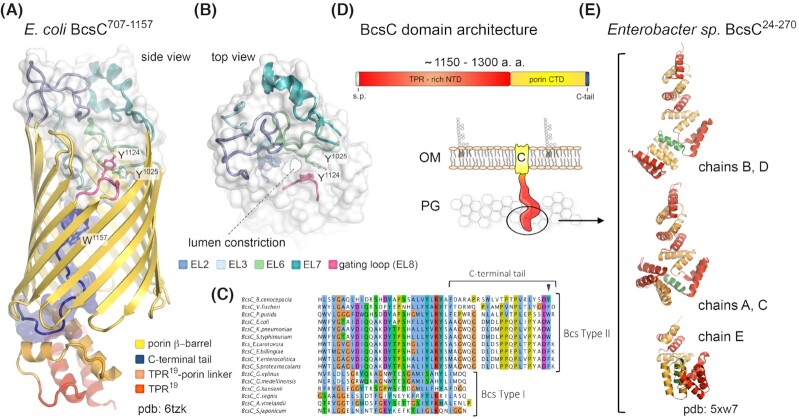
Figure 7.
BcsC and outer membrane cellulose extrusion.(A, B) Crystal structure of E. coli BcsC707-1157 encompassing TPR19 and the outer membrane porin domain (Acheson, Derewenda and Zimmer 2019). The protein is shown in cartoon; the extracellular loops and luminal C-terminal tail are also shown as transparent surface. The lumen constriction proximal to the extracellular surface is seen in panel (B); the gating π-stacking residues Y1025 and Y1124 from extracellular loops EL6 and EL8 are shown as sticks (Acheson, Derewenda and Zimmer 2019). (C) Multiple sequence alignment of BcsC homologs from bacteria featuring Type I and Type II Bcs secretion systems, showing correlation between the luminal C-terminal tail conservation and pEtN-cellulose secretion. (D) BcsC domain architecture and thumbnail representation of the protein in the outer membrane. (E) Crystal structure of the N-terminal TPR modules from an Enterobactersp. BcsC homolog showing multiple conformations between the five chains crystallized in the asymmetric unit (Nojima et al. 2017). TPRs are colored in alternating red and orange; the α5-helix insertion proposed to mediate the conformational flexibility is colored in green.