Figure 8.
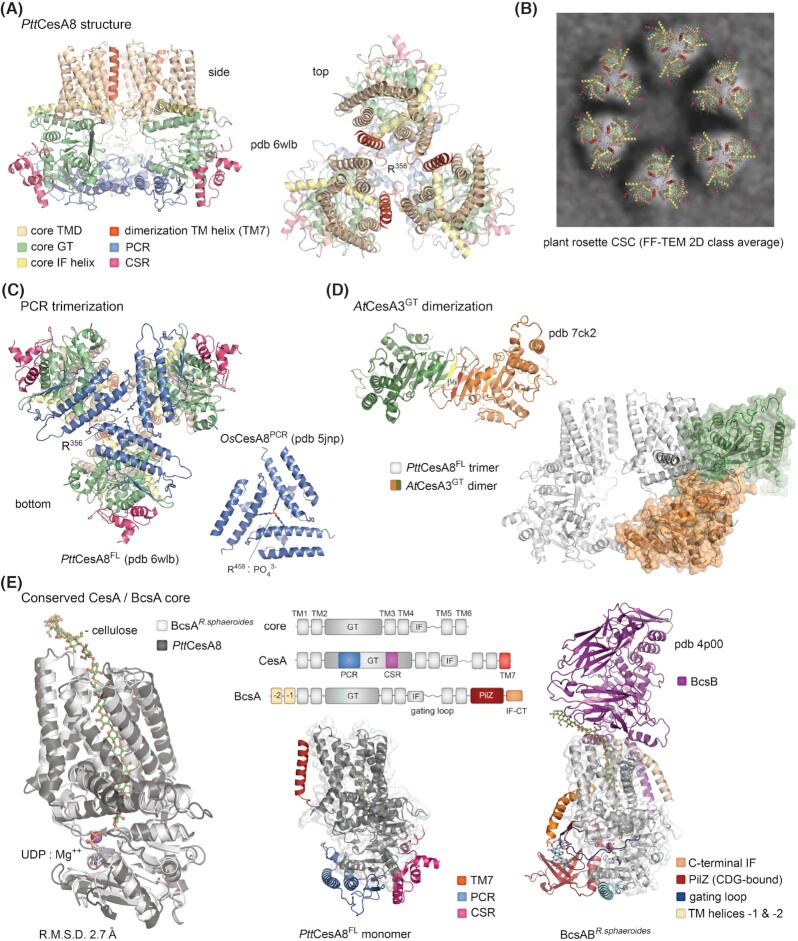
Eukaryotic CesA synthases are structurally conserved Bcs homologs.(A) Cryo-EM structure of the aspen PttCesA8 trimer shown in two different views (Purushotham, Ho and Zimmer 2020). The N-terminal domain is not modeled in the structure. PCR: plant-conserved region; CSR: class-specific region. (B) Proposed structure of the plant CesA rosette complexes based on Nixon et al. (2016) and Purushotham, Ho and Zimmer (2020). The FF-TEM 2D representative view is reproduced under the CC BY 4.0 license (https://creativecommons.org/licenses/by/4.0/legalcode). (C) PCR trimerization in the full-length PttCesA8 protein is virtually identical to that of the isolated PCR domains from rice OsCesA8 (Rushton et al. 2017). Arginine residues partaking in anion/phosphate-based stabilization are shown as sticks. (D) Crystal structure of a dimeric AtCesA3 glycosyl transferase domain based on Qiao et al. (2021). The dimerization β6-strands from each protomer are colored in yellow and red. Bottom right, overlay of the AtCesAGT dimer with the full-length PttCesA8 protein. (E) Conservation of the BcsA/CesA core. Left, overlay of the core transmembrane and glycosyl transferase regions from PttCesA8 and R. sphaeroides BcsA showing virtually identical conformations. Cellulose, UDP and Mg++ are shown as observed in BcsA. Middle top, schematic representation of the conserved synthase core, CesA and BcsA. Middle bottom, structure of the PttCesA8 monomer with the core colored in dark gray and plant-specific insertions in color. Right, structure of the R. sphaeroides BcsAB tandem with the core colored in light gray and bacteria-specific regions in color.