Abstract
Major aortopulmonary collateral arteries (MAPCAs) are congenital vessels that arise from the aorta or its first-order branches and are distally connected to the pulmonary arterial vasculature, thereby providing pulmonary blood flow. MAPCAs are commonly associated with several congenital heart diseases that have compromised pulmonary circulation due to severe stenosis involving pulmonary valves or arteries or due to pulmonary atresia. Embryologically, MAPCAs are presumed to be persistent segmental arteries. MAPCAs can be imaged with CT and MRI, and such imaging findings are important for surgeons and interventionists. The management options for MAPCAs include unifocalization, surgical ligation, and endovascular interventions, such as coil embolization. This review highlights the role of reporting certain critical features of MAPCAs at CT and MRI, which will help to facilitate management decisions for systemic-to-pulmonary collateral vessels observed in patients with congenital heart disease.
Keywords: Pediatrics, CT Angiography, Image Postprocessing, Interventional-Vascular, MR Angiography, Embolization, Stents, Cardiac, Vascular, Aorta
© RSNA, 2022
Keywords: Pediatrics, CT Angiography, Image Postprocessing, Interventional-Vascular, MR Angiography, Embolization, Stents, Cardiac, Vascular, Aorta
Summary
The imaging features of major aortopulmonary collateral arteries and their implications in clinical management are reviewed.
Essentials
■ The extent to which the distal pulmonary vascular bed is supplied by major aortopulmonary collateral arteries (MAPCAs) generally varies inversely to the supply by the true pulmonary arteries.
■ A given lung segment may be supplied solely by the true pulmonary arteries, MAPCAs, or both and show spatial heterogeneity in arterial pressures due to the heterogeneous arborization of MAPCAs.
■ There may be pulmonary over- or undercirculation depending on the presence or absence of communication from MAPCAs and the size of the native pulmonary arteries.
■ Augmented shear stress in segments supplied by nonstenotic MAPCAs causes segmental pulmonary hypertension, while flow-limiting stenosis in the collateral vessels makes the segments oligemic.
■ Patients who have a lung segment with an isolated blood supply from a MAPCA will require either unifocalization, if nonhypertensive, or surgical removal or embolization, if found to be hypertensive, depending on the residual pulmonary anatomy.
■ Nonessential MAPCAs need to be embolized before or after surgery to promote the growth of the intrapericardial pulmonary arteries.
Introduction
Major aortopulmonary collateral arteries (MAPCAs) are congenital heart defects consisting of nonregressed systemic-to-pulmonary embryologic connections from the aorta or its branches to the pulmonary arterial vasculature. MAPCAs augment pulmonary flow in cardiac lesions with compromised antegrade pulmonary arterial blood flow, such as pulmonary atresia with ventricular septal defect (PA-VSD), tetralogy of Fallot, and other cardiac diseases (1). Patients with other complex cardiac abnormalities including unbalanced atrioventricular canal defect, pulmonary atresia with intact ventricular septum, tricuspid atresia, complex transposition, single left ventricle, and double-outlet right ventricle may have a MAPCA if the antegrade pulmonary flow is compromised (2). The prevalence of intraoperatively identified MAPCAs among patients with PA-VSD is 53%–77% (3). Although the pathogenetic mechanisms of MAPCA development are debatable, the postulated hypotheses include hypoxemia, maternal drug intake, diminished global or regional pulmonary blood flow, and nonpulsatile flow in the pulmonary arteries (4–7).
The predominant source of pulmonary blood flow in PA-VSD is either through patent ductus arteriosus or MAPCAs (8). Somerville et al described the anatomy of the native central pulmonary arteries in PA-VSD as very heterogeneous, ranging from valvular pulmonary atresia to total absence of the native central pulmonary arteries, as shown in Figure 1 (9). In PA-VSD, establishing the source and arrangement of the segmental vascular supply of the lung is fundamental for assessing prognosis and surgical management strategies (4,10).
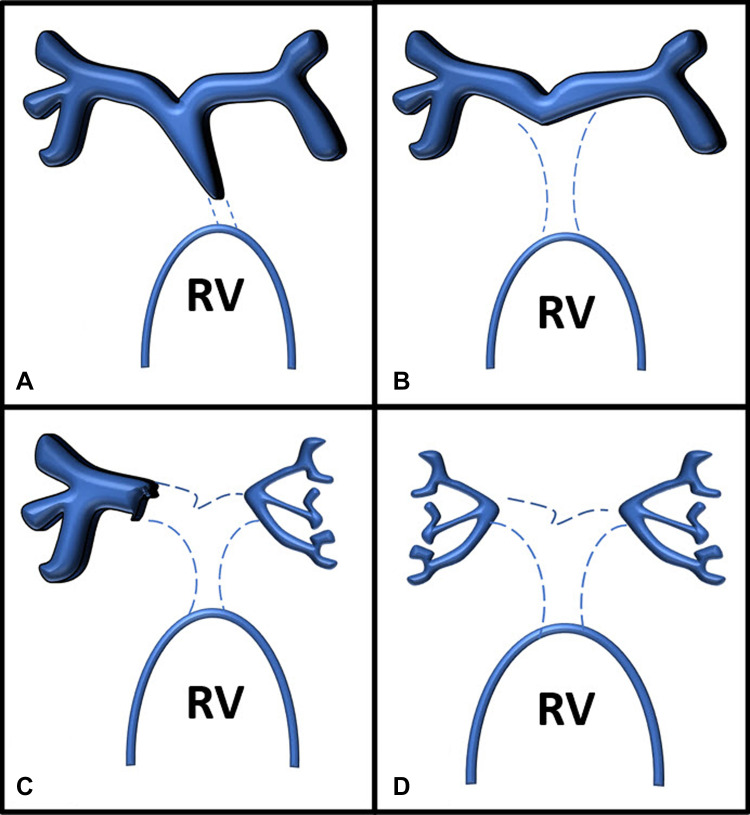
Figure 1:
Somerville classification of pulmonary arterial anatomy in pulmonary atresia with ventricular septal defect. (A) Type 1, atresia of pulmonary valve. (B) Type 2, atresia of pulmonary valve and main pulmonary artery. (C) Type 3, atresia of pulmonary valve, main pulmonary artery, and one main branch. (D) Type 4, atresia of the pulmonary valve, main pulmonary artery, and both main pulmonary artery branches. RV = right ventricle.
A better understanding of the physiology of MAPCAs has come from prior surgical reports (10–12). Findings from these studies have revealed surgical and interventional techniques to accentuate antegrade pulmonary flow, which has led to improved outcomes in patients with MAPCAs. Individualized decisions for clinical management in these cases require inputs from imaging, surgical, and interventional specialists. Cardiac radiologists need to be familiar with these pathologic conditions.
This review provides a brief overview of the embryologic formation of systemic-to-pulmonary collaterals in congenital heart diseases. In addition, this review has a detailed discussion about different imaging modalities and key imaging features for detecting MAPCAs. Last, it discusses various surgical and nonsurgical management options for MAPCAs.
Embryologic Formation
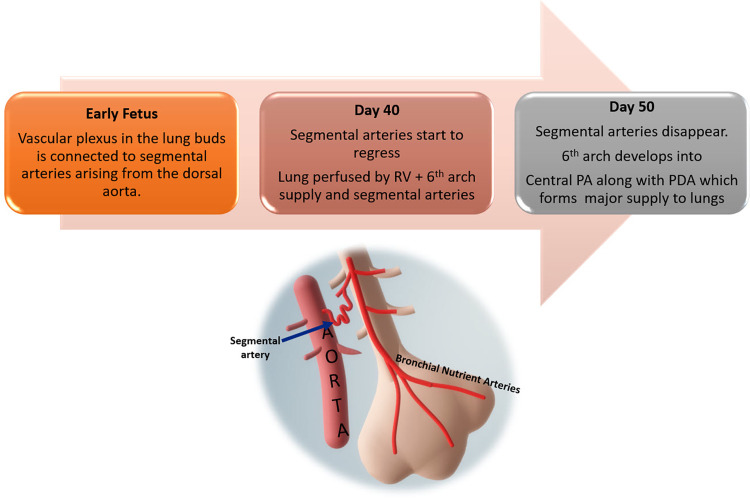
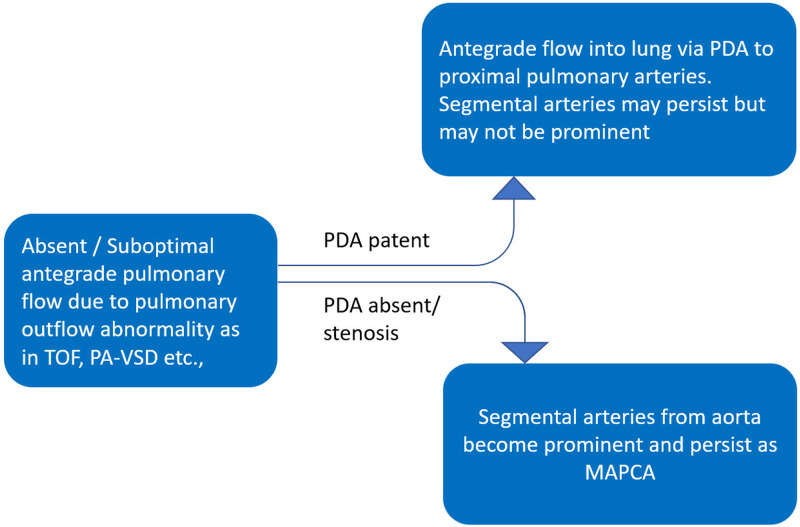
During early fetal development, the vascular plexus of the lung buds is connected to segmental arteries arising from the dorsal aorta. By the 40th day of gestation, the lung receives blood supply from these segmental arteries, as well as from the sixth branchial arch–derived branches (future pulmonary arteries), which are in continuity to the outflow of the right ventricle. By the 50th day of gestation, as the segmental arteries regress, some broncho-pulmonary segments or lobules remain connected to the right ventricle and others to the aorta via the segmental arteries (Fig 2) (13). As the lungs develop, they are exclusively supplied by the central pulmonary arteries (mediastinal main pulmonary artery, right pulmonary artery, and left pulmonary artery) derived from the sixth branchial arches. However, if the pulmonary arteries are not well developed, the ductus arteriosus and segmental arteries from the aorta persist to compensate the pulmonary blood flow, as depicted in Figure 3 (12,13). There are variations in the number, origin, course, branching patterns, and anastomoses of MAPCAs with other pulmonary arteries (such as in Fig 4 with central, lobar, and segmental pulmonary arteries), which may have stenosis at multiple locations for variable lengths (4). MAPCAs may show vasoreactivity and stenosis at multiple locations, reiterating their histologic similarity to the systemic arteries (14).
Figure 2:
Flowchart depicts the normal pulmonary vasculature development with regression of the segmental arteries as the pulmonary artery develops from the sixth branchial arch. PA = pulmonary artery, PDA = patent ductus arteriosus, RV = right ventricle.
Figure 3:
Flowchart shows the development of collateral vessels with regard to the formation of ductus arteriosus. MAPCAs = major aortopulmonary collateral arteries, PA-VSD = pulmonary atresia with ventricular septal defect, PDA = patent ductus arteriosus, TOF = tetralogy of Fallot.
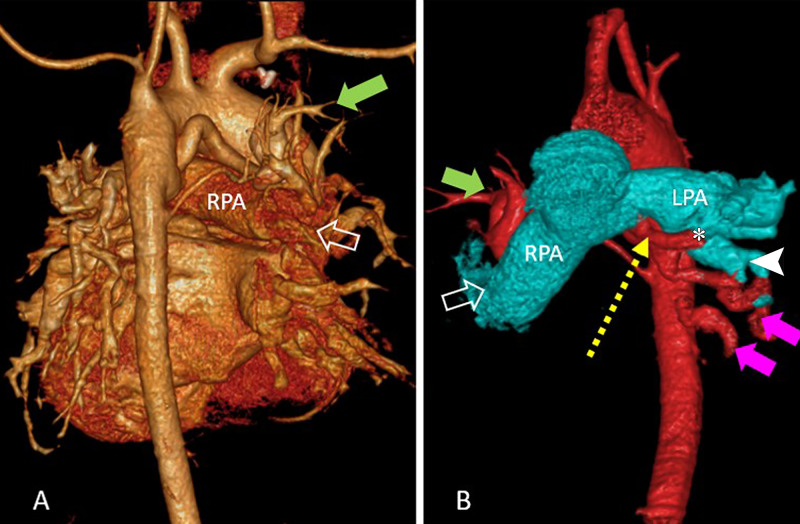
Figure 4:
(A) Volume-rendered reconstruction image in posterior projection shows branching of native pulmonary artery and major aortopulmonary collateral arteries (MAPCAs) in a 9-year-old girl with tetralogy of Fallot and shows predominant supply to right upper lobe from native pulmonary artery (open arrow) along with a MAPCA (green solid arrow). (B) Volume-rendered reconstruction image in anterior projection shows aorta and MAPCA colored in red and pulmonary branches in cyan color with predominant supply of left lower lobe by MAPCA (magenta solid arrows) than by native left pulmonary artery (white arrow head). A communicating collateral (yellow dotted arrow) was observed joining the native left pulmonary artery at hilum from its inferior surface (*). Supply to right upper lobe from native pulmonary artery (open arrow) and MAPCA (green solid arrow) are also labeled.
Types of MAPCA
For ease of understanding the anatomy and physiology, MAPCAs may be classified by three main characteristics: (a) vessel of origin, (b) communication with pulmonary arteries, and (c) impact of stenosis.
Vessel of Origin
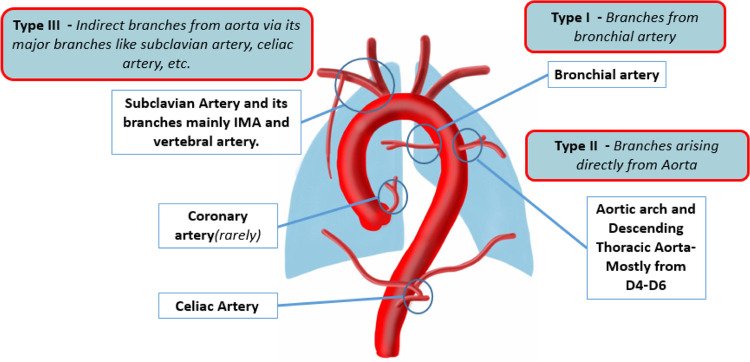
The majority of MAPCAs originate from the anterior wall of the descending thoracic aorta at the level of the carina (1). On the basis of the vessel of origin, MAPCAs are classified as type I, II, or III (Fig 5). MAPCAs may arise from the celiac artery and rarely from the coronary arteries, as shown in Figure 6 (2,4,8,12,13,15).
Figure 5:
Diagram representing the common sites of major aortopulmonary collateral arteries origin and classification. D4–D6 = dorsal vertebrae 4 to 6, IMA = internal mammary artery.
Figure 6:
(A) Volume-rendered CT image in a 10-year-old boy with atrioventricular canal defect and pulmonary stenosis shows the prominent collateral vessels from the left subclavian artery and left internal mammary artery (white dotted arrows). (B) Coronal maximum intensity projection CT image in a 7-year-old boy with double-outlet right ventricle and pulmonary stenosis shows dilated left coronary artery (yellow arrows) supplying the left lung. (C) Digital subtraction angiographic image in same patient as (B) of the celiac artery ostium shows hypertrophied inferior phrenic branches supplying bilateral lung parenchyma (black open arrows).
Communication with Pulmonary Arteries
The second method used to classify MAPCAs is by assessing their connections with other pulmonary arteries. Communicating MAPCAs (ie, nonessential MAPCAs) terminate by forming an anastomosis with distal pulmonary arteries. In these cases, the lung segments may have a dual blood supply (Fig 3). Noncommunicating MAPCAs (ie, essential MAPCAs) form the sole blood supply to the lung segment in the absence of a native pulmonary artery blood supply. A given lung segment may be supplied solely by the true pulmonary arteries, MAPCAs, or both and show spatial heterogeneity in arterial pressures due to the heterogeneous arborization of MAPCAs.
Impact of Stenosis
Last, the effect of stenosis can be used to classify MAPCAs. In the case of nonhypertensive MAPCAs, stenosis will protect the supplied territory from segmental pulmonary hypertension. However, oxygen saturation will be lower than expected from MAPCA-dependent pulmonary circulation. Very thin arteries (<2 mm) are usually nonhypertensive despite the absence of stenosis. In the case of hypertensive MAPCAs, the vessels lack stenosis. Hypertensive MAPCAs are large caliber arteries (>3 mm) demonstrating features of pulmonary arterial hypertension, such as abrupt peripheral tapering and arterial tortuosity resulting from high systemic pressure. These collaterals may lead to MAPCA aneurysms and cause pulmonary hemorrhage if left untreated (16,17).
Pathophysiology of MAPCAs
The pathophysiology of MAPCAs in various congenital heart diseases is attributable to their origin from systemic vessels and the transportation of oxygenated blood to the pulmonary circulation. In unrepaired tetralogy of Fallot or PA-VSD with MAPCAs, the degree of cyanosis varies inversely to the amount of antegrade pulmonary blood flow and the presence of MAPCAs. Interestingly, some patients with MAPCA-dependent pulmonary circulation have increased pulmonary blood flow with higher than normal oxygen saturation; these patients are at risk for developing late pulmonary hypertension or volume overload to the left ventricle, leading to heart failure (16).
The extent to which the distal pulmonary vascular bed is supplied by aortopulmonary collaterals generally varies inversely to that supplied by the true pulmonary arteries (which ranges from being normal in size to being completely absent) (18). There can be pulmonary over- or undercirculation depending on the presence or absence of communication from MAPCAs and the size of the native pulmonary arteries. Over- and undercirculation may coexist in different lung segments of the same patient (especially in PA-VSD). Augmented shear stress in lung segments supplied by nonstenotic MAPCAs causes segmental pulmonary hypertension, while flow-limiting stenosis in the collateral makes the segments oligemic (16). These details are essential for determining the outcome of congenital heart diseases, as explained in the types of MAPCAs and management sections of this article.
Imaging Techniques
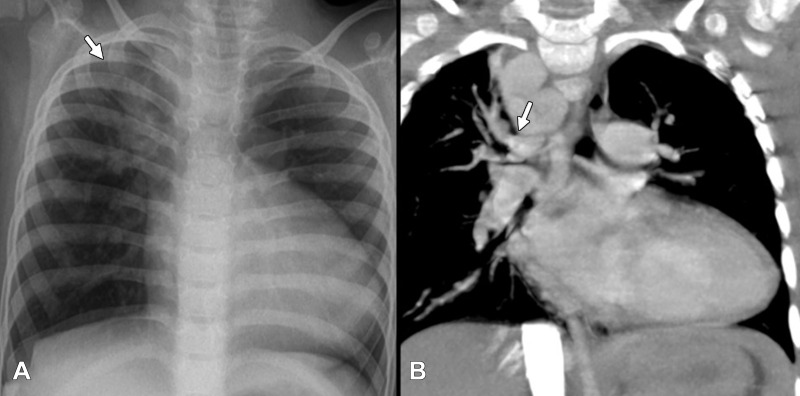
Imaging plays a vital role in evaluating patients who have MAPCAs. Chest radiography is the first-line modality for assessing pulmonary vasculature, lung disorders, and cardiac chamber dilatation. Radiography can depict the difference in pulmonary vascularity between the left and right lung, as well as segmental pulmonary hypervascularity (in segments supplied by collaterals) as shown in Figure 7.
Figure 7:
(A) Chest radiograph in frontal projection of a 4-year-old girl with long segment pulmonary atresia and ventricular septal defect shows markedly increased vascularity involving the right upper zone (white arrow) in comparison with rest of the lung showing normal or mildly reduced vascularity. (B) Correlation with coronal CT image shows dilated major aortopulmonary collateral arteries (white arrow) independently supplying the right upper lobe segments. Child also had a right-sided aortic arch.
Echocardiography can help detect large MAPCAs, particularly those arising from the arch and proximal descending thoracic aorta. However, echocardiography fails in depicting the detailed course, as well as the distal and segmental lung supply. Identifying torrential inflow through pulmonary veins using color Doppler imaging can serve as an indirect marker for collaterals in cyanotic heart diseases, if the central pulmonary arteries are hypoplastic.
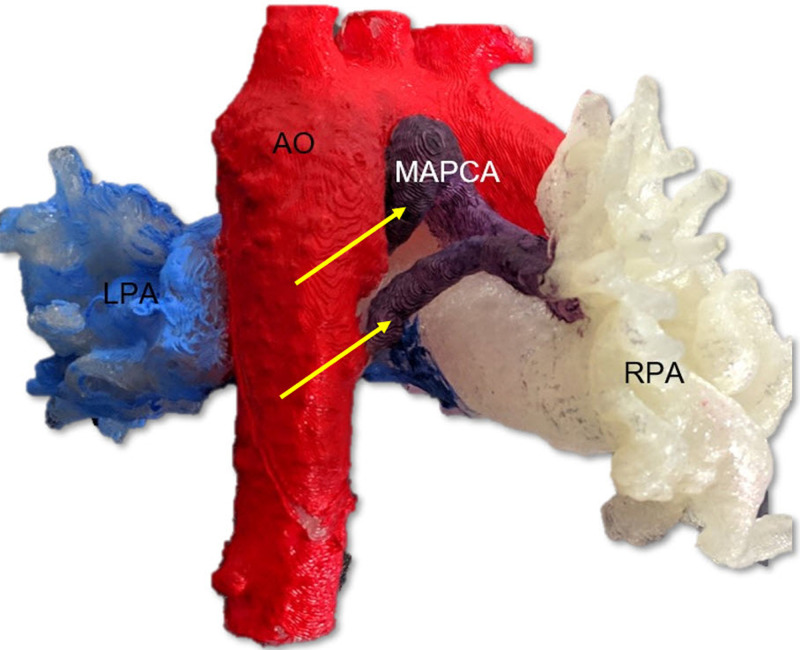
CT imaging can be used to differentiate between essential and nonessential MAPCAs by providing a clear three-dimensional image of the vasculature (Fig 3). CT images can show lung parenchymal changes and location of bronchial collaterals. In addition, assessment of CT images can predict the development of segmental pulmonary hypertension and help to plan selective MAPCA catheterization. In some cases, using CT may obviate using angiography prior to catheterization. In MAPCAs with a predominant posterior mediastinal course, CT scans show the relation of the MAPCA with other mediastinal structures, which is particularly helpful for the surgeon to perform well-planned surgical procedures. Though CT involves radiation exposure, it is considerably lower than that of conventional angiography (19). CT imaging protocols typically include bolus tracking and triggering the study at a threshold of 100 HU in the descending aorta. Multiphase dual-energy CT may prove helpful in depicting differential lung perfusion from MAPCAs and native pulmonary arteries if scans are analyzed for the territory they supply during pulmonary and aortic phases. Three-dimensional printing of the CT data is a useful tool to depict the spatial relationship of patient-specific anatomy, especially of the native pulmonary arteries, aorta, and MAPCA. Three-dimensional printing (Fig 8) provides absolute scaling for planning procedures and for patient education.
Figure 8:
A three-dimensional printed model in right-posterior projection from a patient with pulmonary atresia with ventricular septal defect with major aorto-pulmonary collateral arteries (MAPCA; yellow arrows) arising from the aorta (Ao; colored in red), shown in relation to the right pulmonary arteries (RPA; white color) and left pulmonary arteries (LPA; light blue color), aided in surgical planning prior to unifocalization.
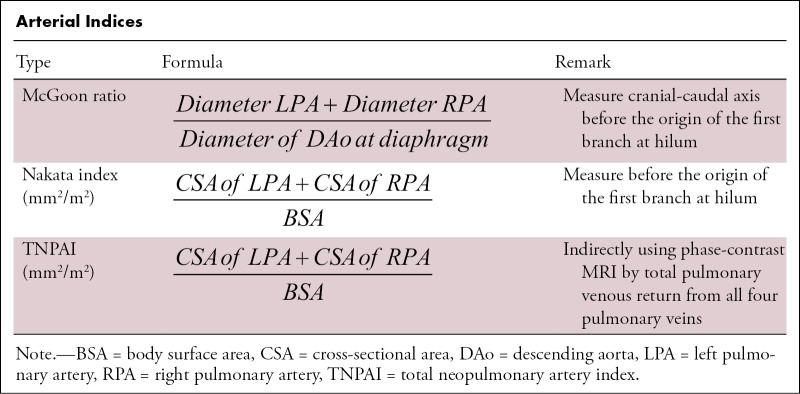
MRI offers the advantage of providing additional functional information (20). The differential flow through the right and left pulmonary arteries (Qp/Qs) and contribution from MAPCAs can be measured with phase-contrast sequences (21). With the new MRI techniques, including centric k-space filling dynamic MR angiography and three-dimensional non–contrast-enhanced sequences, the origin and course of MAPCAs can be identified (Fig 9). Careful assessment of multiphase angiograms shows the varying intensity of contrast material in different vessels (Fig 9) and helps identify the hemodynamics of MAPCAs and pulmonary circulation. However, these sequences require additional validation and refinement before their widespread use is recommended (20,21). A few arterial indexes (as defined in the Table) aid in prognostication and timing of cardiac repair, the details of which are mentioned in the reporting parameters section of this review.
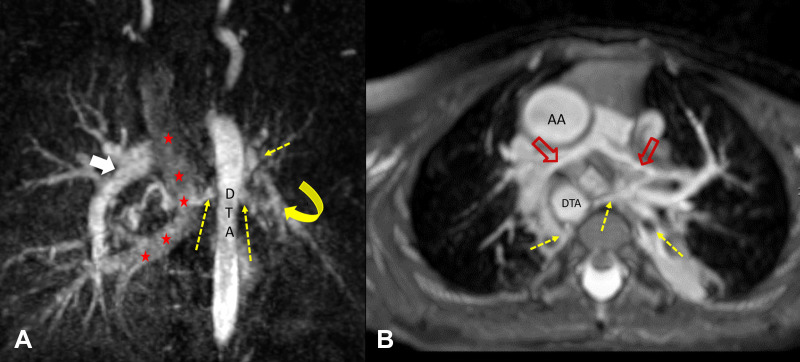
Figure 9:
(A) Coronal maximum intensity projection of multiphase contrast-enhanced MR angiographic image in a 6-year-old girl with total anomalous pulmonary venous return (course marked with red ★s), ventricular septal defect, and hypoplastic left pulmonary artery (yellow curved arrow). Few major aortopulmonary collateral arteries (dotted yellow arrows) arising from the descending thoracic aorta were observed supplying right lower lobe and left upper and lower lobes. The left-sided pulmonary veins were not adequately opacified in this phase but were of normal size in subsequent phases, suggesting slow flow to left lung. The right pulmonary artery was of normal caliber (white arrow). (B) Axial maximum intensity projection of non–contrast-enhanced balanced steady-state free precession angiographic image in a 2-year-old boy with tetralogy of Fallot shows hypoplastic central pulmonary arteries (red open arrows) and multiple major aortopulmonary collateral arteries (yellow dotted arrows) arising from descending thoracic aorta (DTA) directly supplying lung and communicating with native pulmonary artery. AA = ascending aorta.
Arterial Indices
Conventional angiography enables a better depiction of the collaterals and evaluation of hemodynamics. Endovascular interventions can be performed immediately after angiography if indicated. It may be necessary to acquire selective subclavian, celiac, or coronary arterial angiograms to identify certain types of MAPCAs (Fig 6C). Vessel movement in a cine angiogram may be helpful to differentiate mediastinal collaterals from the pulmonary artery branch (intrapericardial pulmonary arteries move with the heart, whereas mediastinal arteries move with the lungs) (22). Measurement of MAPCA pressure should be performed as distally as possible and close to the supplied lung segments before labeling it as a hypertensive MAPCA.
Reporting Parameters
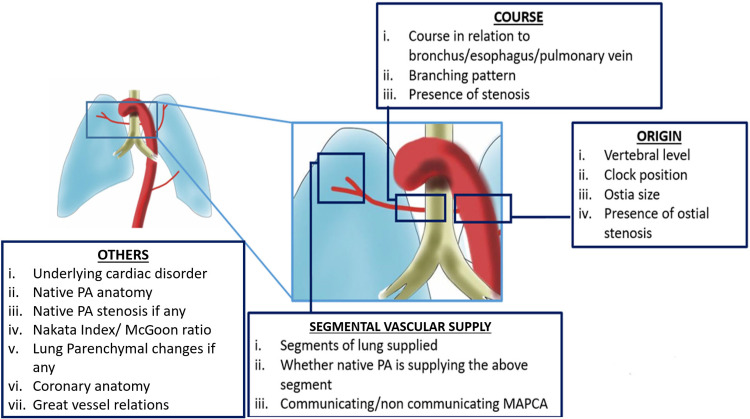
There are a few specific parameters that should be reported at CT when imaging MAPCAs with collaterals (Fig 10): (a) origin, (b) course and branching, (c) stenosis, and (d) segmental vascular supply.
Figure 10:
Summary of the key points that should be included with regard to collateral arteries in the radiology report. MAPCA = major aortopulmonary collateral artery, PA = pulmonary artery.
Origin
Having a record of the vertebral level and the clock position of the collateral origin in the axial plane (from the vantage point of the feet) helps the operating surgeon or interventional radiologist during intervention or unifocalization. Generally, only vessels greater than 3 mm in diameter are considered major collaterals (23,24). Minor collaterals usually are not regarded as clinically significant and do not alter the disease prognosis. Unlike collaterals arising from the descending thoracic aorta, which can be surgically visualized during open-heart surgery, type III MAPCAs (from celiac artery, renal artery, etc) cannot be identified through sternotomy or thoracotomy and will require endovascular management. Hence, radiologists and surgeons must specifically look for these MAPCAs.
Course and Branching
Detailing the course of collaterals in relation to the esophagus and tracheobronchial tree will help the operating surgeon to easily identify these vessels (3). The branching pattern of the collaterals is important as these vessels can have multiple branches to multiple segments, even to both lungs. Different branches of the same collateral could be supplying a segment either as a communicating MAPCA or independently, which would determine the type of management for each collateral.
Stenosis
Common locations of stenosis include the origin, at the junction with pulmonary arteries, retroesophageal location, and at branching points (3). Origin stenosis will be protective for the lung segment against the development of pulmonary hypertension. Stenosis in branches or distal insertion of MAPCAs need specific mention because they are important causes of failure of intracardiac repair after unifocalization requiring patch repair during surgery. The length of stenosis and involvement of branching points are necessary to know prior to unifocalization surgeries. Additionally, the presence of ostial stenosis provides interventional radiologists with additional information regarding the success rate of cannulation of the collateral during angiography.
Segmental Vascular Supply
For an excellent postoperative outcome, the maximum number of lung segments must be perfused by the native pulmonary arterial branches (13). Hence, it is crucial to describe the detailed anatomy of blood vessels supplying the lung segments as independently or jointly supplied by native pulmonary branches or MAPCAs, as shown in Figure 3. An isolated supply of a lung segment by MAPCA will require either unifocalization, if nonhypertensive, or surgical removal or embolization, if found hypertensive, depending on the residual pulmonary anatomy. There are also multiple CT-derived indexes for assessing the adequacy of the pulmonary circulation, such as the McGoon ratio and Nakata index (Table) (25,26). The need for early surgical shunts or right ventricle outflow tract stents can be decided on the basis of a McGoon ratio cutoff value of less than 1.2 or total neopulmonary artery index of less than 100, both of which indicate a small-caliber central pulmonary artery (27,28). A McGoon ratio of more than 1.5 suggests optimal pulmonary vascular resistance (ie, adequacy of the pulmonary vascular bed to accommodate the cardiac output without causing heart failure). Normal Nakata index values are around 330 mm2/m2 ± 30 (standard deviation). Generally, patients with Nakata indexes of greater than 150 mm2/m2 who undergo biventricular repair have higher survival rates (29).
Imaging Analogs of MAPCAs
Multiple anatomic structures appear similar to MAPCAs at CT and should be carefully considered when assessing imaging findings.
Patent Ductus Arteriosus
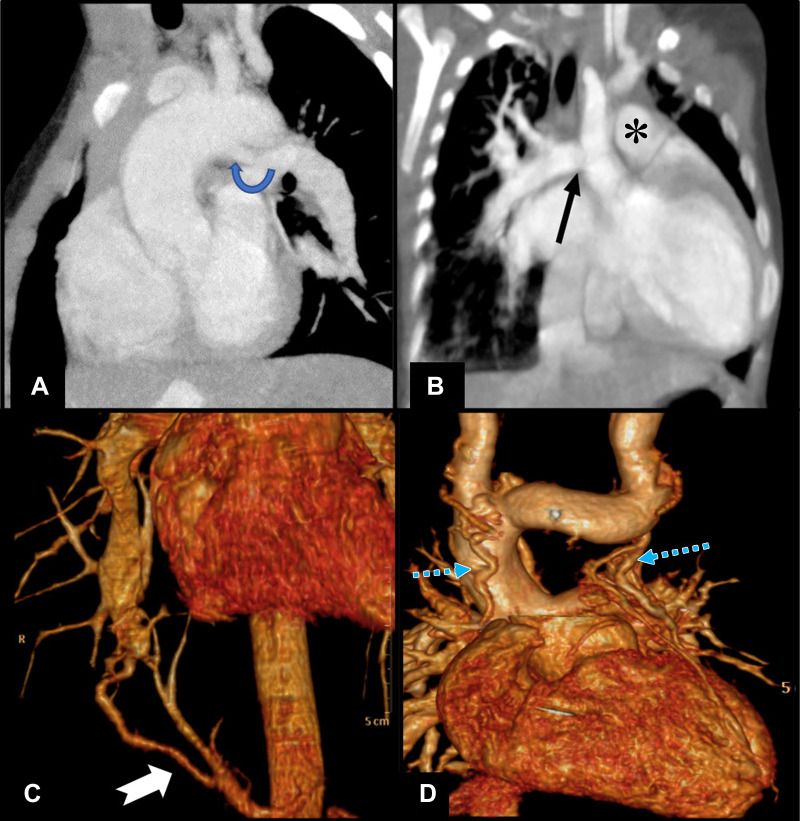
MAPCAs are commonly confounded with patent ductus arteriosus, which originates from the undersurface of the aortic arch, distal to the subclavian origin, and course to join the proximal pulmonary artery without any intervening branches (Fig 11A). Patent ductus arteriosus is also associated with a narrowing at the attachment site to the branch pulmonary artery and is less tortuous and shorter in course. However, both patent ductus arteriosus and MAPCAs may coexist in the same patient. Unusual patent ductus arteriosus from a subclavian artery can cause diagnostic dilemma; however, absence of branching in the mediastinum before joining the pulmonary artery favors patent ductus arteriosus (1).
Figure 11:
(A) Sagittal oblique cardiac CT section in a 1-year-old boy shows patent ductus arteriosus (rounded arrow) draining into left pulmonary artery in a case of pulmonary atresia and ventricular septal defect. (B) Coronal oblique cardiac CT image shows right pulmonary artery (black arrow) arising from aorta in a 4-month-old girl, suggestive of hemitruncus. There was coexisting main pulmonary artery (*) supply to left lung. (C) Volume-rendered cardiac CT image shows isolated pulmonary supply from aorta (white tailed arrow) to lung parenchyma in a 26-year-old man who presented with hemoptysis and no structural heart disease, suggestive of type B malinosculation. (D) Coronal projection of CT angiographic image in volume-rendered reconstruction (excluding anterior ascending aorta for visibility) in a 7-year-old boy with double-outlet right ventricle after staged hemi-Fontan surgery shows small vessels in mediastinum with tortuous courses (blue dotted arrows) having communication with superior caval vein or its tributaries and veins draining into azygous vein, suggestive of venovenous collaterals.
Hemitruncus
Hemitruncus, wherein one side of the pulmonary artery arises from the ascending aorta while the other arises as a continuation main pulmonary artery (Fig 11B), can be mistaken for a large MAPCA (30). However, compared to hemitruncus, MAPCAs, in general, do not originate from the ascending aorta.
Hypertrophied Bronchial Artery
A hypertrophied bronchial artery mimicking a MAPCA can be recognized by its relation to the trachea and bronchial tree and how it forms a nutritive plexus in the bronchial walls.
Type B Malinosculation
MAPCAs can be confounded with type B malinosculation (Fig 11C), where a lung segment is supplied by an independent systemic arterial branch from the aorta with or without anomalous bronchial tree communication. Compared with MAPCAs, malinosculation is not associated with a cardiac anomaly or reduced pulmonary artery size. Type B malinosculation typically affects the left lower lobe. The pulmonary arterial supply to the involved segment may be present or absent, but the venous return is always through the inferior pulmonary vein.
Acquired Collateral Arteries
Acquired collateral arteries (pseudosequestration) can also be confounded with MAPCAs. Acquired collateral arteries develop after pleural or lung surgeries when adhesion of the visceral and parietal pleural layers creates centripetal collateral circulation from the chest wall to the lungs. Acquired collateral arteries appear at aortography as myriads of tiny vessels, which may originate from an artery in the thorax, including intercostal arteries. MAPCAs are typically easily differentiated from acquired collateral arteries.
Venovenous Collaterals
Venovenous collaterals observed in patients after bidirectional Glenn surgery (Fig 11D) must be differentiated from MAPCAs for further clinical management. The difference in attenuation of the contrast material in the vessels in comparison with aortic branches and tracing both ends of the vessel can be used to distinguish between venovenous collaterals and MAPCAs. Usually, venovenous collaterals will have a lower Hounsfield unit value than an adjacent MAPCA when looked at during the arterial phase of cardiac CT.
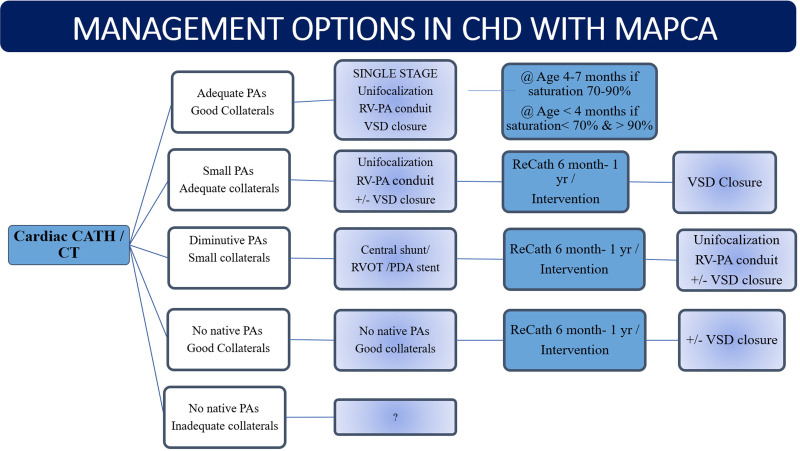
Management Options
The goal of management in patients with MAPCAs is to treat cardiac conditions such as VSD, tetralogy of Fallot, and pulmonary atresia so that the right ventricle can pump blood to both lung segments. A brief algorithm for management is shown as a flowchart in Figure 12. As MAPCAs have high pressures due to their origin from the systemic circulation, patients with MAPCAs can develop pulmonary hypertension or hemorrhage if not adequately managed. Treatment has evolved from palliative shunting or interruption of the collateral arteries (as performed in the 1970s) to unifocalization of the pulmonary arteries connected to the right ventricle with or without closure of the ventricular septal defect (10,31,32). Unifocalization includes the direct reimplantation of the origin of a MAPCA from the aorta to the native or reconstructed pulmonary artery.
Figure 12:
Algorithm for surgical management of congenital heart disease (CHD) in presence of major aortopulmonary collateral arteries (MAPCAs). A Nakata index of less than 100 mm2/m2 or McGoon ratio of less than 1.2 is considered for central shunt creation or right ventricle outflow tract or patent ductus arteriosus (PDA) stent placement. A reduced Qp/Qs of less than 1 is an indication for staging ventricular septal defect (VSD) closure. CATH = catheter, PA = pulmonary artery, Qp/Qs = differential flow through right and left pulmonary arteries, RV = right ventricle, RVOT = RV outflow tract.
Haworth et al postulated that blood flow to the lungs might be normalized by unifocalizing the individual arteries with the intrapericardial pulmonary arteries (25). Operative therapy is currently recommended for all patients whose general condition and anatomic findings are amenable to surgical repair (11).
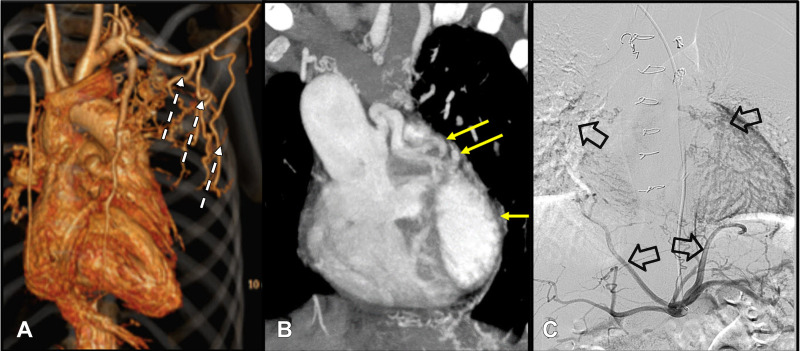
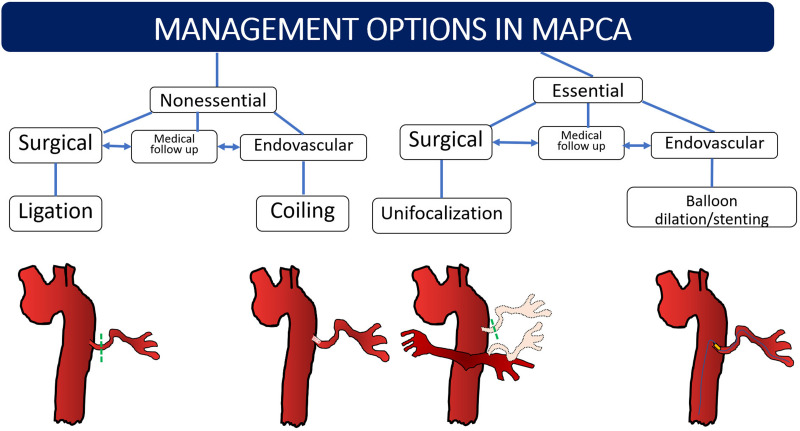
The presence of a large nonessential MAPCA could lead to clinically significant hypoperfusion syndrome following the initiation of cardiopulmonary bypass. During aortic cross-clamp with bypass, the excessive return of blood to the left heart could lead to distention of the ventricle and impaired myocardial protection. The excessive return of blood will obscure the operative field. Also, pulmonary edema can cause difficulty in extubating the patient after the procedure (33). Collaterals that are likely to be directly visualized in the surgical field can be managed with ligation. However, collaterals from subclavian and celiac arteries will require endovascular management. Nonessential MAPCAs need to be embolized before or after surgery to promote the growth of the intrapericardial pulmonary arteries. Requisites of endovascular embolization include a diameter of 3 mm or larger, a collateral causing recurrent hemoptysis as shown in Figure 13A, and substantial volume load to the systemic ventricle. Materials such as tissue adhesives, vascular plugs, and Gianturco coils have been used to embolize collateral arteries (33). Currently, coils are most commonly used for this purpose (Fig 13B). Cross-sectional imaging or simultaneous pulmonary angiography needs to be performed to ensure an alternative source of arterial supply to the lung segments before the collaterals are occluded. The coil is usually oversized to 30%–50% larger than that of the target artery (33).
Figure 13:
(A) CT and (B) digital subtraction angiographic images in a 14-year-old boy with tetralogy of Fallot and associated pulmonary infundibular stenosis. The child presented with hemoptysis with lung parenchyma showing areas of pulmonary hemorrhage (red arrow in A) in right lung. (B) Descending thoracic aortic angiographic image shows the major aortopulmonary collateral arteries (MAPCAs) arising at D6 level that were embolized using vascular coils (black dotted arrow). (C) Coronal oblique projection of cardiac CT angiographic image in an 8-year-old boy with pulmonary atresia with ventricular septal defect shows a large collateral vessel from right subclavian artery (white solid arrow) supplying right upper lobe. The descending segment of this collateral vessel was narrowed and had a stent placed (yellow arrow) to maintain saturation, as central pulmonary arteries were poorly developed.
Essential MAPCAs need to be salvaged by disconnecting these arteries from the aorta and anastomosing to the main branches of native pulmonary arteries through unifocalization (Fig 14), which should be performed before stenosis or hypertension develops as the child ages. Unifocalization can be performed by direct anastomosis or by the placement of interposition grafts (27). Definitive intracardiac repair has favorable results if 15 of the 20 bronchopulmonary segments are connected to the pulmonary circulation from the right ventricle (13). A Nakata index of less than 150 mm2/m2 or Qp/Qs of less than 1 at CT or MRI indicates the need for unifocalization or staging the definitive surgery (11,21). The role of the radiologist is in identifying and describing in detail the segmental arterial supply of both the lungs so that the surgeon can decide which segments would need unifocalization. Segments that receive both native pulmonary arterial branch and systemic collateral (such as in Fig 4A) need not be unifocalized, but the collateral may be surgically ligated or embolized endovascularly. In patients who are ineligible for surgery, balloon dilation and stent placement of stenotic essential MAPCAs (Figs 13–14) can be performed as palliative procedures (24). Medical follow-up is advised in cases of stenotic nonessential MAPCAs or when a child is waiting for a definitive or palliative procedure.
Figure 14:
Summary of management options in major aortopulmonary collateral arteries (MAPCAs) in general. Essential MAPCAs require either unifocalization or endovascular stent placement or angioplasty. Nonessential and dilated MAPCAs are coiled or ligated. Nonessential stenosed MAPCAs may be kept under medical follow-up before or after surgery and addressed later. If all essential MAPCAs cannot be unifocalized in a single stage, the remaining MAPCAs are followed-up until the next stage of repair.
Summary
The presence of MAPCAs is a major determining factor in the prognosis of various congenital heart diseases involving pulmonary outflow abnormalities. The role of a cardiac radiologist is in reporting critical components regarding the collateral vessels and underlying cardiac structural disease. With endovascular management offering excellent results, and owing to the simplicity of the procedure compared with open surgical ligation, it is now considered the standard of care in patients requiring occlusion of the collaterals. MRI with flow study has the potential for deriving hemodynamic data and hence may replace angiographic assessment for management.
Authors declared no funding for this work.
Disclosures of Conflicts of Interest: A. Alex No relevant relationships. A. Ayyappan No relevant relationships. J.V. No relevant relationships. H.K. No relevant relationships. D.S. No relevant relationships. S.M. No relevant relationships.
Abbreviations:
- MAPCA
- major aortopulmonary collateral artery
- PA-VSD
- pulmonary atresia with ventricular septal defect
- Qp/Qs
- differential flow through right and left pulmonary arteries
References
- 1. Wernovsky G , Anderson RH , Kumar K , et al. , eds. Anderson’s Pediatric Cardiology . 4th ed. Philadelphia, Pa: : Elsevier; , 2019. ; 660 – 663 . [Google Scholar]
- 2. Marino B , Calabró R , Gagliardi MG , Bevilacqua M , Ballerini L , Marcelletti C . Patterns of pulmonary arterial anatomy and blood supply in complex congenital heart disease with pulmonary atresia . J Thorac Cardiovasc Surg 1987. ; 94 ( 4 ): 518 – 520 . [PubMed] [Google Scholar]
- 3. Ryan JR , Moe TG , Richardson R , Frakes DH , Nigro JJ , Pophal S . A novel approach to neonatal management of tetralogy of Fallot, with pulmonary atresia, and multiple aortopulmonary collaterals . JACC Cardiovasc Imaging 2015. ; 8 ( 1 ): 103 – 104 . [DOI] [PubMed] [Google Scholar]
- 4. Seale AN , Ho SY , Shinebourne EA , Carvalho JS . Prenatal identification of the pulmonary arterial supply in tetralogy of Fallot with pulmonary atresia . Cardiol Young 2009. ; 19 ( 2 ): 185 – 191 . [DOI] [PubMed] [Google Scholar]
- 5. Pelizzo G , Calcaterra V , Mannarino S , Moramarco LP , Leati G , Quaretti P . Aortopulmonary collateral artery in prenatal exposure to carbamazepine - endovascular therapy and technical considerations: a case report . J Med Case Rep 2015. ; 9 183 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boshoff D , Gewillig M . A review of the options for treatment of major aortopulmonary collateral arteries in the setting of tetralogy of Fallot with pulmonary atresia . Cardiol Young 2006. ; 16 ( 3 ): 212 – 220 . [DOI] [PubMed] [Google Scholar]
- 7. Bauser-Heaton H , Borquez A , Han B , et al . Programmatic Approach to Management of Tetralogy of Fallot With Major Aortopulmonary Collateral Arteries: A 15-Year Experience With 458 Patients . Circ Cardiovasc Interv 2017. ; 10 ( 4 ): e004952 . [DOI] [PubMed] [Google Scholar]
- 8. Sato Y , Ogino H , Hara M , et al . Embolization of collateral vessels using mechanically detachable coils in young children with congenital heart disease . Cardiovasc Intervent Radiol 2003. ; 26 ( 6 ): 528 – 533 . [DOI] [PubMed] [Google Scholar]
- 9. Murthy KS , Rao SG , Naik SK , Coelho R , Krishnan US , Cherian KM . Evolving surgical management for ventricular septal defect, pulmonary atresia, and major aortopulmonary collateral arteries . Ann Thorac Surg 1999. ; 67 ( 3 ): 760 – 764 . [DOI] [PubMed] [Google Scholar]
- 10. Rammeloo LAJ , DeRuiter MC , van den Akker NM , Wisse LJ , Gittenberger-de Groot AC . Development of major aorto-pulmonary collateral arteries in vegf120/120 isoform mouse embryos with tetralogy of fallot . Pediatr Cardiol 2015. ; 36 ( 1 ): 89 – 95 . [DOI] [PubMed] [Google Scholar]
- 11. Thiene G , Frescura C , Bini RM , Valente M , Gallucci V . Histology of pulmonary arterial supply in pulmonary atresia with ventricular septal defect . Circulation 1979. ; 60 ( 5 ): 1066 – 1074 . [DOI] [PubMed] [Google Scholar]
- 12. Bailliard F , Anderson RH . Tetralogy of Fallot . Orphanet J Rare Dis 2009. ; 4 ( 1 ): 2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sasikumar D , Sasidharan B , Ayyappan A , Gopalakrishnan A , Krishnamoorthy KM . Coronary-to-pulmonary artery collaterals in pulmonary atresia . Ann Pediatr Cardiol 2018. ; 11 ( 3 ): 328 – 329 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Somerville J , Saravalli O , Ross D . Complex pulmonary atresia with congenital systemic collaterals . Classification and management. Arch Mal Coeur Vaiss 1978. ; 71 ( 3 ): 322 – 328 . [PubMed] [Google Scholar]
- 15. Griselli M , McGuirk SP , Winlaw DS , et al . The influence of pulmonary artery morphology on the results of operations for major aortopulmonary collateral arteries and complex congenital heart defects . J Thorac Cardiovasc Surg 2004. ; 127 ( 1 ): 251 – 258 . [DOI] [PubMed] [Google Scholar]
- 16. Amin Z , McElhinney DB , Reddy VM , Moore P , Hanley FL , Teitel DF . Coronary to pulmonary artery collaterals in patients with pulmonary atresia and ventricular septal defect . Ann Thorac Surg 2000. ; 70 ( 1 ): 119 – 123 . [DOI] [PubMed] [Google Scholar]
- 17. Dimopoulos K , Diller GP , Opotowsky AR , et al . Definition and Management of Segmental Pulmonary Hypertension . J Am Heart Assoc 2018. ; 7 ( 14 ): e008587 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rodefeld MD , Reddy VM , Thompson LD , et al . Surgical creation of aortopulmonary window in selected patients with pulmonary atresia with poorly developed aortopulmonary collaterals and hypoplastic pulmonary arteries . J Thorac Cardiovasc Surg 2002. ; 123 ( 6 ): 1147 – 1154 . [DOI] [PubMed] [Google Scholar]
- 19. Wang RP , Liang CH , Huang MP , Liu H , Deng QP , Yang MF . Assessment of aortopulmonary collateral flow and pulmonary vascular growth using a 3.0 T magnetic resonance imaging system in patients who underwent bidirectional Glenn shunting . Eur J Cardiothorac Surg 2012. ; 41 ( 6 ): e146 – e153 . [DOI] [PubMed] [Google Scholar]
- 20. Grosse-Wortmann L , Yoo SJ , van Arsdell G , et al . Preoperative total pulmonary blood flow predicts right ventricular pressure in patients early after complete repair of tetralogy of Fallot and pulmonary atresia with major aortopulmonary collateral arteries . J Thorac Cardiovasc Surg 2013. ; 146 ( 5 ): 1185 – 1190 . [DOI] [PubMed] [Google Scholar]
- 21.Anderson RH, Seo JW, Ho SY. The pulmonary arterial supply in tetralogy of Fallot with pulmonary atresia. In: Yacoub MH, Pepper JR,eds.Annual of cardiac surgery 1990–1991. London, England:Current Science,1991;77–83. [Google Scholar]
- 22. Babliak OD , Mykychak YB , Motrechko OO , Yemets IM . Surgical treatment of pulmonary atresia with major aortopulmonary collateral arteries in 83 consecutive patients . Eur J Cardiothorac Surg 2017. ; 52 ( 1 ): 96 – 104 . [DOI] [PubMed] [Google Scholar]
- 23. Doshi D , Oswal N , Shukla A , Patel P , Shah K . Clinical study of incidence of significant major aorto pulmonary collateral arteries in patients of TOF and its correlation with pulmonary artery anatomy . Pediatr Rev Int J Pediatr Res 2017. ; 4 ( 2 ): 100 – 105 . [Google Scholar]
- 24. Mainwaring RD , Patrick WL , Carrillo SA , Ibrahimye AN , Muralidaran A , Hanley FL . Prevalence and Anatomy of Retroesophageal Major Aortopulmonary Collateral Arteries . Ann Thorac Surg 2016. ; 102 ( 3 ): 877 – 882 . [DOI] [PubMed] [Google Scholar]
- 25. Haworth SG , Macartney FJ . Growth and development of pulmonary circulation in pulmonary atresia with ventricular septal defect and major aortopulmonary collateral arteries . Br Heart J 1980. ; 44 ( 1 ): 14 – 24 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Piehler JM , Danielson GK , McGoon DC , Wallace RB , Fulton RE , Mair DD . Management of pulmonary atresia with ventricular septal defect and hypoplastic pulmonary arteries by right ventricular outflow construction . J Thorac Cardiovasc Surg 1980. ; 80 ( 4 ): 552 – 567 . [PubMed] [Google Scholar]
- 27. Nakata S , Imai Y , Takanashi Y , et al . A new method for the quantitative standardization of cross-sectional areas of the pulmonary arteries in congenital heart diseases with decreased pulmonary blood flow . J Thorac Cardiovasc Surg 1984. ; 88 ( 4 ): 610 – 619 . [PubMed] [Google Scholar]
- 28. Reddy VM , Petrossian E , McElhinney DB , Moore P , Teitel DF , Hanley FL . One-stage complete unifocalization in infants: when should the ventricular septal defect be closed? J Thorac Cardiovasc Surg 1997. ; 113 ( 5 ): 858 – 866 . discussion 866–868 . [DOI] [PubMed] [Google Scholar]
- 29. Fraentzel O . Ein Fall von abnormer Communication der Aorta mit der Arteria pulmonalis . Arch Pathol Anat Physiol Klin Med 1868. ; 43 ( 3 ): 420 – 426 . [Google Scholar]
- 30. Patrick WL , Mainwaring RD , Reinhartz O , Punn R , Tacy T , Hanley FL . Major Aortopulmonary Collateral Arteries With Anatomy Other Than Pulmonary Atresia/Ventricular Septal Defect . Ann Thorac Surg 2017. ; 104 ( 3 ): 907 – 916 . [DOI] [PubMed] [Google Scholar]
- 31. Sawatari K , Imai Y , Kurosawa H , Isomatsu Y , Momma K . Staged operation for pulmonary atresia and ventricular septal defect with major aortopulmonary collateral arteries . New technique for complete unifocalization. J Thorac Cardiovasc Surg 1989. ; 98 ( 5 Pt 1 ): 738 – 750 . [PubMed] [Google Scholar]
- 32. Bhalgat P , Naik A , Someshwar V , Joshi S . Unmasking the culprit: MAPCA masquerading as RV failure in post surgical correction of TOF . J Cardiol Cases 2016. ; 15 ( 3 ): 95 – 96 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brühlmann W , Weishaupt D , Goebel N , Imhof E . Therapeutic embolization of a systemic arterialization of lung without sequestration . Eur Radiol 1998. ; 8 ( 3 ): 355 – 358 . [DOI] [PubMed] [Google Scholar]