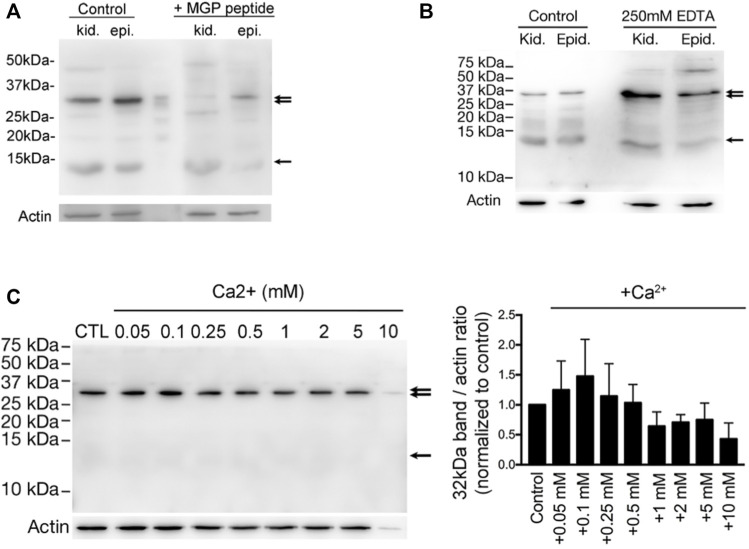
FIGURE 3.
Vitamin K-dependent MGP-mediated calcium-promoted aggregation of a protein complex with a prominent band of ∼32-kDa. (A) Western blot detection of anti-MGP in total homogenates of kidney (kid.) and epididymis (epi.) from WT adult rats. A band at ∼12-KDa (arrow) corresponding to the expected molecular size of MGP, and another major band at around ∼32-kDa (double arrow) were detected. Both bands were almost abolished by the preincubation with a ten-fold excess of the MGP immunizing peptide (+MGP peptide). (B) The intensity of ∼32-kDa bands (double arrow) were significantly enriched in the low-Ca2+ condition by addition of 250 mM EDTA to the protein lysates, whereas the ∼12-kDa bands remained unchanged (arrow). Some bands at higher molecular sizes became obvious under the low-Ca2+ condition. (C) The same anti-MGP antibody was used to detect the intensity changes of ∼32-kDa band in DC2 cell protein lysates under various Ca2+ concentrations and the bar graph on the right shows the intensity of the band normalized to control (no additional Ca2+). This suggests that MGP-mediated protein-aggregation is dependent on sub-millimolar amount of Ca2+, whereas excessive Ca2+ (>0.25 mM) inhibits protein-aggregation. (Originally published in iScience (Ma et al., 2019), with permission to reproduce from iScience).