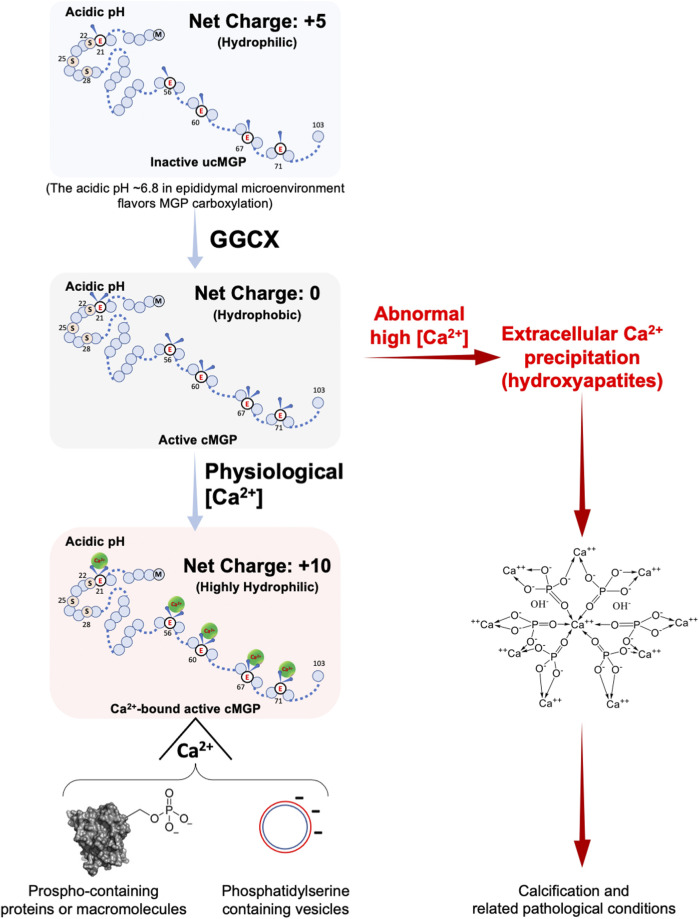
FIGURE 4.
Calcium-dependent chelation property of MGP with or without carboxylation. MGP is a protein composed of 103 amino acids and can be post-translationally modified by carboxylation and phosphorylation. Uncarboxylated MGP (ucMGP) is an inactive form with an isoelectric point of around 9 (8.8 for rat and 9.6 for human MGP), which means that in an acidic environment, the ucMGP has a net positive charge of five, despite its five γ-glutamate residues, and thus it is hydrophilic. When the five γ-glutamate residues are carboxylated to γ-carboxylglutamates by GGCX enzymatic activity, the carboxylated MGP (cMGP) becomes active but is electrically neutral and thus hydrophobic. Intriguingly, the five carboxylated γ-glutamate residues of cMGP bear five negative charges, which favors the chelation of five Ca2+ ions and form a highly hydrophilic ten-positively-charged cMGP-Ca2+ compound, facilitating chelation of negative-moiety-bearing molecules under normal physiological conditions, particularly aggregation of organic or inorganic phosphates, or the membrane-embedded phosphatidylserine such as membranes of extracellular vesicles. Dysregulation of the Ca2+-binding property of cMGP results in ectopic Ca2+ precipitation and abnormal Ca2+ content, which causes cMGP to bind with hydroxylapatites and thereby precipitation nucleation, a precursor status of promoting matrix calcification and related pathological conditions.