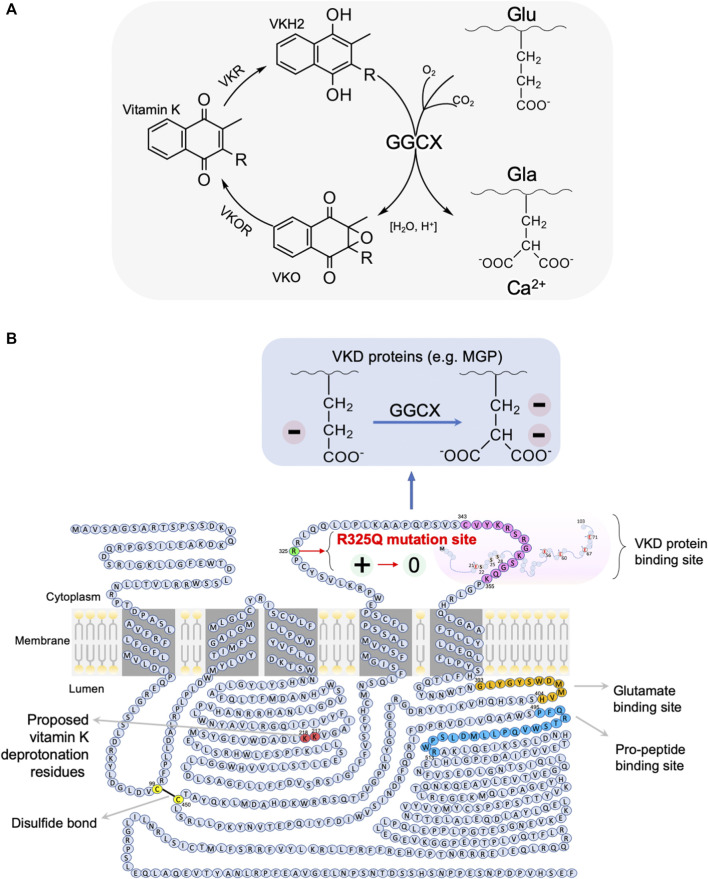
FIGURE 5.
A mechanistic model for the association of the GGCX arginine325 to glutamine325 (R325Q) SNP with asthenozoospermia infertility risk in men. (A) Vitamin K cycle: during vitamin K dependent (VKD) carboxylation, the carboxylase GGCX mediates the pH-dependent conversion of glutamate (Glu) residues to Ca2+-binding gamma-carboxyglutamate (Gla) residues of the vitamin K-dependent substrates (e.g., MGP) or their pro-peptides, with CO2, O2 and reduced vitamin K as the co-substrates. The carboxylation reaction also oxidises the reduced vitamin K-H2 to vitamin K epoxide (VKO), which is then reduced back to vitamin K-H2 by vitamin K epoxide reductase (VKOR). The cycle involves redox- and pH-sensitive carboxylation and oxygenation processes (Rishavy and Berkner, 2012). (B) GGCX is a membrane protein with a size of ∼87.5 kDa, which has two intracellular loops and two luminal loops of amino acid sequences, in addition to its cytoplasmic N-terminus and a long luminal C-terminus. The binding sites for glutamate and pro-peptide are located on the long C-terminal tail, which can form a disulfide bond with the first luminal loop of amino acids. It has been hypothesized that all five Glu residues of MGP are carboxylated in one round in sequential order by GGCX, presumably due to MGP stabilisation by the singly positively charged arginine325 of GGCX (Rishavy and Berkner, 2012). In our proposed model, the rs699664 SNP-mediated change of arginine325 to a neutral glutamine325 moiety in the GGCX leads to the loss of GGCX binding stability to MGP for carboxylation, thus resulting in decreased carboxylation of VKD proteins, and thereby to increased calcium mineralization and Ca2+-mediated proliferation of stress granules, and eventually in a disordered epididymal luminal microenvironment, causing impaired sperm maturation and male infertility. Graphic illustration is adapted from (Tie et al., 2016) with modifications. For more details, see the main text.