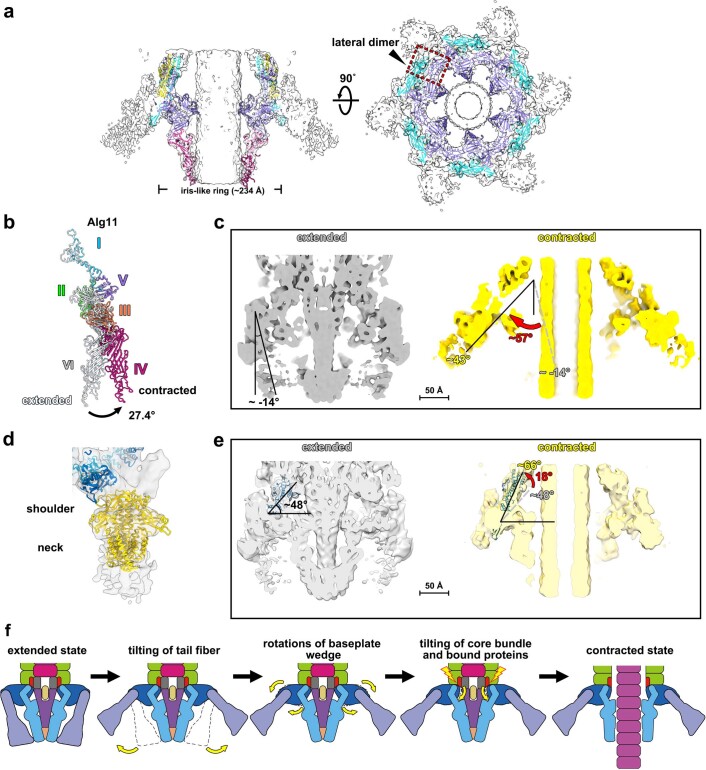
Extended Data Fig. 8. Conformational changes upon firing reveal the fate of the AlgoCIS cage.
a: Side view (left) and bottom view (right) of shadowed surface and ribbon diagrams, showing that the spike cage remains attached to the baseplate and opens. The iris-like ring is intact in contracted AlgoCIS. The color code matches Fig. 4a. The red dashed box indicates the position of one lateral dimer. b: Structural superpositions of Alg11 proteins in the extended and contracted states showing that the conformational change of domain IV mainly contributes to the opening of spike cage. The color code for different domains in the contracted state matches Fig. 4b, while the structure of Alg11 in the extended state is colored white. c: Cutaway view of shadowed surface diagrams of extended (grey) and contracted (yellow) AlgoCIS structures showing that the tail fiber has a large outward tilt upon firing. The extended and contracted maps were both lowpass-filtered to 10 Å. Bar: 50 Å. d: Shadowed surface and ribbon diagram showing that the docking of the T6SS TssK structure [gold, PDB entry: 5MWN42] fits the overall structure of the AlgoCIS tail fiber. The tail fiber has a similar shape as the shoulder domain in TssK. e: Cutaway views of shadowed surface and ribbon diagrams of extended (grey) and contracted (yellow) AlgoCIS structures showing that the baseplate core bundles have a higher tilt angle related to the plane of the iris-like ring after contraction. The extended and contracted maps were both lowpass-filtered to 10 Å. Bar: 50 Å. f: Schematic showing the putative contraction and signal transmission process in AlgoCIS upon firing. The color code matches Fig. 1d. The lightning bolts represent the conformational changes of the gp25-like protein Alg9, which triggers sheath contraction.