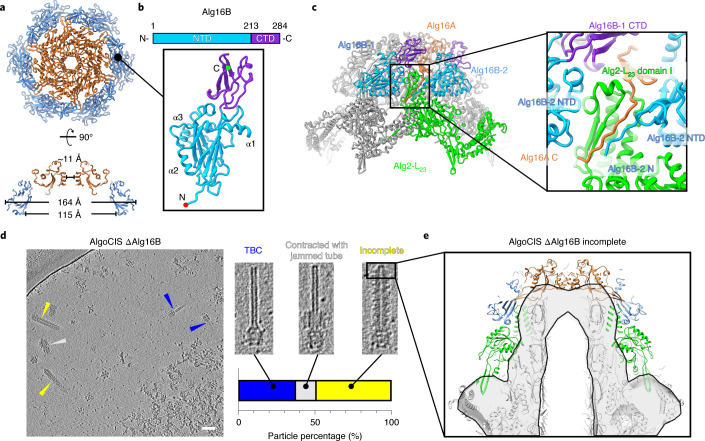
Fig. 2. Cap and cap adaptor proteins terminate AlgoCIS.
a, Top view (top) and side view (bottom) of ribbon diagrams showing that cap proteins (Alg16A) interact with cap adaptor proteins (Alg16B) and together assemble into the cap module. The colour code matches Fig. 1d. b, Schematic and ribbon diagrams showing that the cap adaptor protein (Alg16B) has two domains. The N- and C-terminal domains (NTD and CTD) are cyan and purple, while the N and C termini are marked with red and green circles. c, Left: ribbon diagrams of cap module showing that Alg16A and Alg16B adopt a mimicked hand-shaking manner to terminate the sheath-tube module. The colour code for one individual protomer of Alg16A and distal sheath layer (Alg2-L23) matches Fig. 1d, whereas two protomers of cap adaptor protein (Alg16B-1 and Alg16B-2) are coloured on the basis of the domain organization in b. The box indicates the interactions between cap module and sheath protein. Right: the structural detail of the box on the left. d, CryoET of AlgoCIS ΔAlg16B particles reveals three different classes of aberrant particles. Shown is an overview image, three examples for the different classes, and a quantification (11 nm thick tomographic slices). Scale bar, 50 nm. e, Sub-tomogram averaging of the AlgoCIS ΔAlg16B ‘incomplete’ class from d, revealing that the cap, cap adaptor and the distal sheath layer are absent. Shown is a comparison of the wild-type model (ribbon diagram) and the average (grey and black). The proteins absent in AlgoCIS ΔAlg16B are colour-coded according to Fig. 1d.