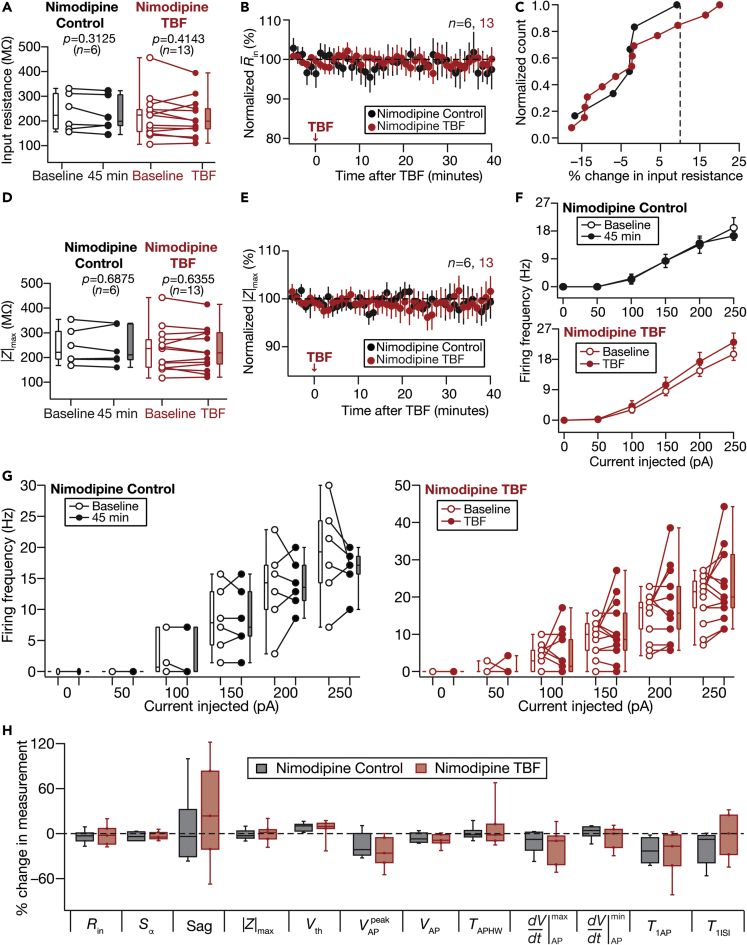
Figure 10.
Activity-dependent intrinsic plasticity was dependent on calcium influx into the cytosol through L-type calcium channels
Control (Black) and TBF (red) group experiments were performed in the presence of 10-μM Nimodipine in the bath. The Control groups (black) correspond to experiments where no protocol was applied through the 45-min period of the experiment, and TBF group (red) is for neurons subjected to TBF, with all experiments performed in the presence of Nimodipine.
(A) Population data representing change in Rin at the beginning (empty circles) and end (filled circles) of the experiment. Two-way mixed ANOVA, interaction p = 0.897
(B) Temporal evolution of percentage changes in (mean ± SEM)
(C) Normalized count of neurons from panel A plotted as functions of percentage change in Rin
(D and E) Same as panels A–B, representing |Z|max measurements. Two-way mixed ANOVA, interaction p = 0.917
(F) Summary statistics (mean ± SEM) of action potential firing frequency plotted as a function of injected current amplitude for both groups. ∗p <0.05; ∗∗p <0.005. Student’s t test
(G) Population data representing changes in the action potential firing frequency at the beginning (empty circles) and end (filled circles) of the experiment, for six values of current injection, for the Control (left) and TBF (right) groups. For each of the five (50, 100, 150, 200, and 250 pA) current injections, there was no significant interaction between time (0 vs. 45 min) and protocol (Control vs. TBF) factors when assessed with two-way mixed ANOVA
(H) Plots comparing percentage change in various measurements from their baseline-to-final values are provided for each measurement. The Wilcoxon signed rank test was used for p value calculation in panels A and D, for comparing measurements from the same set of cells. The Wilcoxon rank-sum test was employed for p-value calculation in panel H, to compare percentage changes in the Control vs. TBF group