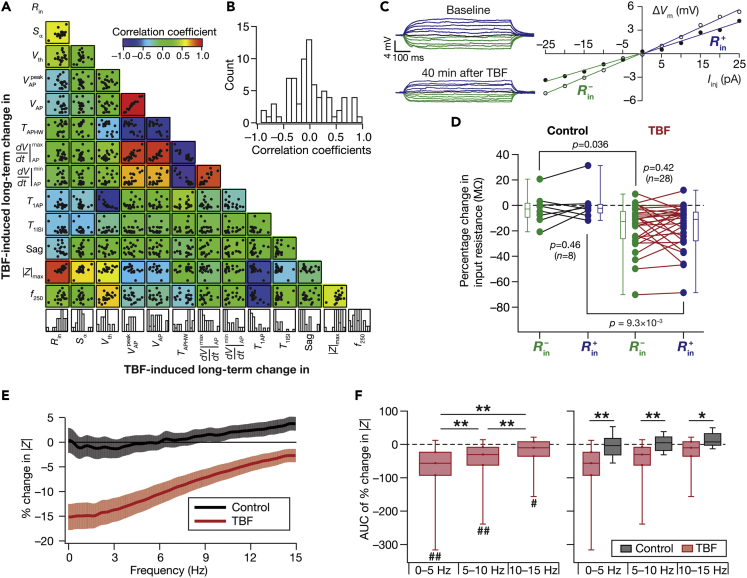
Figure 4.
Signature changes in several physiological measurements point to TBF-induced changes in multiple ion channels, with plasticity in resting conductances as a candidate mechanism behind the reduction in sub-threshold excitability
(A and B) Differential pair-wise correlations among TBF-induced changes in 13 electrophysiological measurements. (A) Pairwise scatterplot matrices of TBF-induced changes in 13 sub- and supra-threshold measurements recorded from granule cells (n = 20). These scatterplot matrices are overlaid on the corresponding color-coded correlation matrices. The bottom most row presents the histogram of percentage changes for corresponding measurements spanning their respective ranges. Symbols: input resistance, Rin; summation ratio of αEPSPs, Sα; AP threshold, Vth; AP peak, ; AP amplitude, VAP; AP half-width, TAPHW; peak dV/dt, ; min dV/dt, ; latency to first spike, T1AP; first ISI, T1ISI; percentage sag, Sag; Maximal impedance amplitude, |Z|max; firing frequency for a 250-pA current injection, f250. (B) Distribution of correlation coefficient values for 13 measurements corresponding to the pairwise scatterplots shown in panel (A).
(C–F) Analyses of changes in steady-state and frequency-dependent measurements point to HCN channels as a candidate mechanism behind TBF-induced reduction in sub-threshold excitability. (C) Voltage responses of an example neuron to 700 ms current pulses of amplitude varying from −25 pA to +25 pA (in steps of 5 pA), recorded before (Baseline) and 40 min after TBF. The responses colored blue and green are for positive and negative current injections, respectively. Input resistance values, computed from depolarizing and hyperpolarizing responses, were slopes of the plots depicting steady-state voltage responses as functions of positive and negative current injections, respectively (bottom). were calculated for traces obtained before (open circles) and after (closed circles) TBF (bottom). (D) Population level analysis to quantify percentage changes (at the end of experiment, compared to the measurement at the beginning) in (blue) and (green) independently for Control (n = 8) and TBF (n = 28) experiments. The p-values presented for comparisons within each group (either Control or TBF) correspond to Wilcoxon signed rank test and p-values across groups are for Wilcoxon rank-sum test. Two-way mixed ANOVA, interaction p = 0.563. (E) Percentage changes in impedance amplitude as a function of frequency for Control (no protocol) and TBF groups. In the Control group, the percentage changes were computed as the change in impedance amplitude measured at 45 min, compared to the initial measurements. In the TBF group, the percentage changes are between those measured before and 40 min after TBF. (F) Quantification of percentage changes in |Z| within three frequency bands (0–5, 5–10, and 10–15 Hz). The value for each neuron were computed as the AUC of percentage change in |Z|. Left: plot showing the quartiles of AUC of percentage change in |Z| for neurons in the TBF group. The symbol “#” refers to the outcomes of Wilcoxon rank-sum test on whether TBF-induced changes in |Z| within that frequency band were significantly different from zero. #p <0.05; ##p <0.001. The symbol “∗” refers to the outcomes of Wilcoxon signed rank test, assessing whether TBF-induced changes in |Z| across the different frequency bands were significantly different from one another. ∗∗p <0.001. Right: plot showing the quartiles of AUC of percentage change in |Z| for neurons in the Control and TBF groups. The symbol “∗” refers to the outcomes of Wilcoxon rank-sum test, assessing whether TBF-induced changes in |Z| within each frequency band were significantly different between the Control and TBF groups. ∗p <0.05; ∗∗p <0.005. For the Control group, changes in |Z| within none of the three frequency bands were significantly different from zero, or significantly different from one another. The representation has been split into two separate graphs to avoid clutter of symbols denoting statistical test outcomes