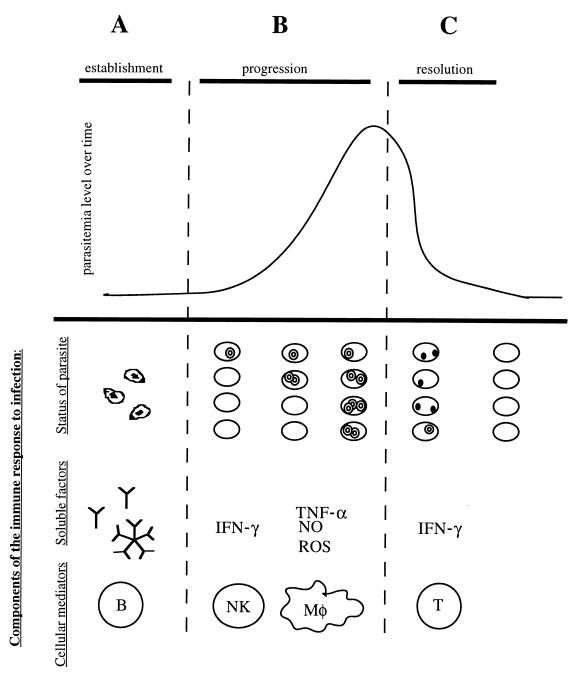
FIG. 3.
Theoretical model of the cells and molecules involved in immunity to Babesia species. Different immune mechanisms contribute to resistance during each stage of babesial infection. During the establishment stage (A), antibodies (IgG) play a role in preventing erythrocyte infection by binding the free sporozoites. During this progression stage (B), the Babesia organisms succeed in invading the erythrocyte, and the resulting merozoites start proliferating and lyse the infected cell. After lysis has occurred, parasites reach the bloodstream again to initiate a new round of invasion. Several rounds of this cycle cause the overall parasitemia level to increase. Cells of the innate immune system are thought to control the growth rate of the merozoites and therefore the rate of increasing parasitemia. Specifically, NK cells and macrophages have been implicated in antibabesial activity. The inhibition seems to rely on the production of soluble factors: IFN-γ by NK cells and TNF-α, nitric oxide (NO), and ROSs by macrophages (Mφ). The specific mechanism of protection, however, remains unclear. In the resolution stage (C), parasitemia levels in babesiosis usually reach a maximum and then decline. The decrease in parasite numbers seems to be due at least in part to intracellular degeneration inside the erythrocyte, as evidenced by the appearance of crisis forms. T-cell lymphocytes seem to be the cells responsible for parasite clearance, specifically the subpopulation of CD4+ IFN-γ producers. The mechanism of parasite eradication and its relation to IFN-γ production remain unknown.