Abstract
Accumulating evidence indicates that inflammation and nutrition status are associated with clinical outcomes in patients with various malignancies. This study aimed to evaluate the prognostic significance of the pretreatment platelet to albumin ratio (PAR) in esophageal squamous cell carcinoma (ESCC) patients undergoing definitive radiotherapy. A total of 470 patients who underwent definitive radiotherapy with or without chemotherapy were enrolled. The optimal cut-off values of PAR and other indicators were determined by the X-tile. The Kaplan–Meier method, multivariate analyses Cox regression were conducted to identify the association between those indicators and the survival outcomes. The median follow-up time was 23.5 months. The optimal cut-off value of PAR was 5.7 × 109 and patients were stratified as the low PAR group and the high PAR group. In the univariate analysis, a low overall survival rate was significantly associated with T stage (P = 0.005), TNM stage (P < 0.001), Adjuvant chemotherapy (P = 0.007), neutrophil to lymphocyte ratio (NLR) (P = 0.006), platelet to lymphocyte ratio (P < 0.001), systemic immune-inflammation index (P < 0.001), prognostic nutritional index (P < 0.001) and platelet to albumin ratio (PAR) (P < 0.001). Patients with high PAR were associated with poorer OS and PFS than patients with low PAR. On multivariate analysis, TNM stage (P = 0.001), adjuvant chemotherapy (P < 0.001), and PAR (P = 0.033) were independent prognostic factors in ESCC treated with definitive radiotherapy. PAR is a novel, convenient, and inexpensive prognostic indicator for patients with ESCC undergoing definitive radiotherapy. Future validation from prospective larger-scale studies is warranted.
Subject terms: Cancer, Biomarkers
Introduction
Esophageal cancer (EC) is one of the fatal tumor types throughout the world1. In China, EC is still a severe public health problem with high morbidity and mortality2. The majority of patients are diagnosed at advanced stages and lose the probability of curative resection. Despite the fact that alternative treatments such as immunotherapy and molecular-targeted therapy have been booming over the last decade, the clinical outcomes remain unsatisfactory1,3. Therefore, the identification of reliable indicators for prognosis prediction and individualized risk stratification has become a trending topic in the practice of EC research.
Accumulating evidence has revealed that nutritional status, cancer-related inflammation and the immune system play a vital role in carcinogenesis, proliferation, progression, and metastasis4,5. Several inflammation-based prognostic indicators such as the neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), and systemic immune-inflammation index (SII) were demonstrated as independent prognostic factors in various solid tumors, including esophageal cancer, lung cancer, gastric cancer, colorectal cancer, and prostate cancer6–10. Several nutrition-based indicators including the prognostic nutritional index (PNI) are also known as critical prognostic elements in cancer patients. Published investigations have suggested a poor nutritional status was associated with a worse prognosis in various malignancies11–13. PAR comprises both platelet count and serum albumin concentration and is applied to assess the inflammation-nutrition status. Several studies have reported that PAR could serve as independent prognostic markers in some types of cancers, including lung cancer14, hepatocellular cancer15, Pancreatic cancer16, and cholangiocarcinoma17. However, the relationship between the pretreatment PAR and the survival of patients with esophageal squamous cell carcinoma (ESCC) remains uncertain.
To the best of our knowledge, the prognostic role of PAR in ESCC patients who received definitive radiotherapy has not been reported. Therefore, in the current study, we aimed to evaluate whether the pretreatment PAR was associated with the clinical outcomes and could serve as an independent prognostic factor in ESCC patients undergoing definitive radiotherapy.
Materials and methods
Patients
Patients who underwent definitive radiotherapy for esophageal cancer were retrospectively reviewed from January 2011 to December 2015 at our center. The inclusive criteria were as follows: (I) histologically confirmed as ESCC; (II) Karnofsky score ≥ 70; (III) treated with definitive radiotherapy (50–70 Gy in 25–35 fractions, 5 days per week) with 0–6 cycles of platinum-based chemotherapy; (IV) complete clinicopathological and pretreatment serum laboratory data; (V) neither the history of malignancy nor acute and/or chronic inflammatory disease and (VI) restaged according to the 8th edition of the American Joint Committee on Cancer TNM staging system for esophageal cancer18. The study was approved by the the Ethics Committee of the Fujian Medical University Cancer Hospital. Written informed consent was waived by the the Ethics Committee of the Fujian Medical University Cancer Hospital due to the retrospective nature of the study. All methods were performed in accordance with relevant guidelines and regulations (the ethical standards of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards).
Treatment protocol
Radiotherapy was delivered by two-dimensional conventional radiotherapy (2D-CRT), three-dimensional conformal radiation therapy (3D-CRT), or intensity-modulated radiation therapy (IMRT) technique in this study. The gross tumor volume (GTV) was determined by the contrast-enhanced CT, barium swallow, endoscopic examination, or PET-CT, which contained both the primary tumor and the positive regional lymph nodes. The clinical target volume (CTV) is composed of subclinical lesions (GTV extending 0.5–1.0 cm in axial direction and 3 cm in longitudinal direction) and the relative mediastinal lymphatic drainage field. The CTV plus a 0.5 cm expansion margin in all directions was defined as the planning target volume (PTV). Patients who received two-dimensional conventional radiotherapy (2DRT) used anterior and posterior opposing techniques. The irradiated field included the primary tumor and a distal and proximal margin of 3 cm and a 0.5–1.0 cm radial margin around the tumor.
The Platinum-based two-drug chemotherapy regimen was used for some enrolled patients: (I) paclitaxel 135 mg/m2 D1 or docetaxel 75 mg/m2 D1 + cisplatin or nedaplatin 75 mg/m2 D2; (II) 5-fluorouracil (5-FU) 700–1000 mg/m2 D1–2 + cisplatin 75 mg/m2 D2, every three weeks as a cycle.
Definition of the NLR, PLR, SII, PNI and PAR
The baseline neutrophil, lymphocyte, platelet counts, and serum albumin levels were collected from routine test reports 7 days prior to the first treatment. The definitions of NLR, PLR, SII, PNI and PAR are calculated as follows:
NLR = absolute neutrophil count/absolute lymphocyte count;
PLR = platelet counts/absolute lymphocyte count;
SII = platelet counts* absolute neutrophil count/absolute lymphocyte count;
PNI = serum albumin level (g/L) + 5* absolute lymphocyte count;
PAR = platelet counts/serum albumin level (g/L).
The optimal cutoff values for the NLR, PLR, SII, PNI and PAR were determined by X-tile software (http://www.tissuearray.org/rimmlab)19.
Follow-up
During treatment, patients were assessed weekly to screen the treatment toxicities, including blood routine, biochemical test and physical examination. After treatment, Follow-up examinations were conducted 1 month after finishing radiotherapy, and then every 3 months in the first year, every 6 months over the next 2 years, and once a year thereafter. The routine examination items included physical examination, laboratory tests, tumor markers, thoracic CT scanning, and esophageal barium. The followed up lasted until death or the last contact. The primary endpoint was overall survival (OS), which was defined as the period from treatment initiation to the date of last follow-up or death from any cause. Progression-free survival (PFS) was defined as the period from treatment initiation to the date of disease progression or death from any cause. Disease progression was evaluated by the standard Response Evaluation Criteria in Solid Tumors20.
Statistical analysis
All Statistical analysis was performed with SPSS 26.0 (SPSS, Chicago, IL). The association between the PAR groups and clinicopathological characteristics was analyzed by the χ2 test. Survival curves for OS and PFS were plotted via the Kaplan–Meier method and compared by the log-rank test. Univariate and multivariate cox analyses were conducted to identify the independent risk or prognostic factors. A P value < 0.05 was considered as statistically significant.
Result
Patient characteristics
The baseline clinicopathological characteristics of 470 patients in the study were shown in Table 1. All patients consisted of 333 (70.9%) men and 137 (29.1%) women, with the median age of 64 years (range: 36–94 years). 43 (9.1%), 109 (23.2%), 271 (57.7%), and 47 (10.0%) patients had primary tumors located in the cervical, upper, middle, and lower thoracic esophagus respectively. Based on the criteria of the 8th edition AJCC TNM staging system, 95 (20.2%), 142 (30.2%), and 233 (49.6%) of cases were diagnosed as stage II, stage III, and stage IV. Thirty-two patients received 2D-CRT, and 438 patients received 3D-CRT and IMRT. Besides, there were 321 (68.3%) patients who received adjuvant chemotherapy.
Table 1.
Baseline clinical variables of the study participants stratified by pretreatment PAR.
| Variables | Total, n(%) | Low PAR (N = 172) | High PAR (N = 298) | P-value |
|---|---|---|---|---|
| Gender | 0.007 | |||
| Male | 333 (70.9) | 109 | 224 | |
| Female | 137 (29.1) | 63 | 74 | |
| Age | 0.020 | |||
| ≤ 70 | 305 (64.9) | 100 | 205 | |
| > 70 | 165 (35.1) | 72 | 93 | |
| Location | 0.124 | |||
| Cervical | 43 (9.1) | 22 | 21 | |
| Upper third | 109 (23.2) | 41 | 68 | |
| Middle third | 271 (57.7) | 96 | 175 | |
| Lower third | 47 (10.0) | 13 | 34 | |
| T stage | 0.003 | |||
| T2 | 43 (9.1) | 19 | 24 | |
| T3 | 217 (46.2) | 94 | 123 | |
| T4 | 210 (44.7) | 59 | 151 | |
| N stage | 0.084 | |||
| N0 | 118 | 51 | 67 | |
| N + | 352 | 121 | 231 | |
| cTNM stage | 0.001 | |||
| II | 95 (20.2) | 46 | 49 | |
| III | 142 (30.2) | 59 | 83 | |
| IV | 233 (49.6) | 67 | 166 | |
| RT technique | 0.158 | |||
| 2D-CRT | 32 (6.8) | 8 | 24 | |
| IMRT + 3D-CRT | 438 (93.2) | 164 | 274 | |
| Chemotherapy | 0.031 | |||
| No | 149 (31.7) | 65 | 84 | |
| Yes | 321 (68.3) | 107 | 214 | |
| Marrow depression | 0.568 | |||
| No | 224 (47.7) | 79 | 145 | |
| Yes | 246 (52.3) | 93 | 153 | |
| NLR | 0.297 | |||
| < 2.62 | 275 (58.5) | 106 | 169 | |
| ≥ 2.62 | 195 (41.5) | 66 | 129 | |
| PLR | < 0.001 | |||
| < 180 | 364 (77.4) | 156 | 208 | |
| ≥ 180 | 106 (22.6) | 16 | 90 | |
| SII | < 0.001 | |||
| < 577.7 | 254 (54.0) | 131 | 123 | |
| ≥ 577.7 | 216 (46.0) | 41 | 175 | |
| PNI | 0.499 | |||
| < 41.5 | 64 (13.6) | 21 | 43 | |
| ≥ 41.5 | 406(86.4) | 151 | 255 |
PAR platelet to albumin ratio, NLR neutrophil to lymphocyte ratio, PLR platelet to lymphocyte ratio, SII systemic immune-inflammation index, PNI prognostic nutritional index, 2DRT two-dimensional conformal radiation therapy, 3DRT three-dimensional conformal radiation therapy, IMRT intensity-modulated radiation therapy.
The optimal cutoff values for the NLR, PLR, SII, PNI and PAR calculated by the X-tile was 2.62, 180, 577.7, 41.5, and 5.7 × 109, respectively.(Supplementary Figs. 1 and 2) Then patients were divided into the low PAR group (PAR < 5.7 × 109) and the high PAR group (PAR ≥ 5.7 × 109) for further analyses.
Association between PAR and clinicopathological characteristics
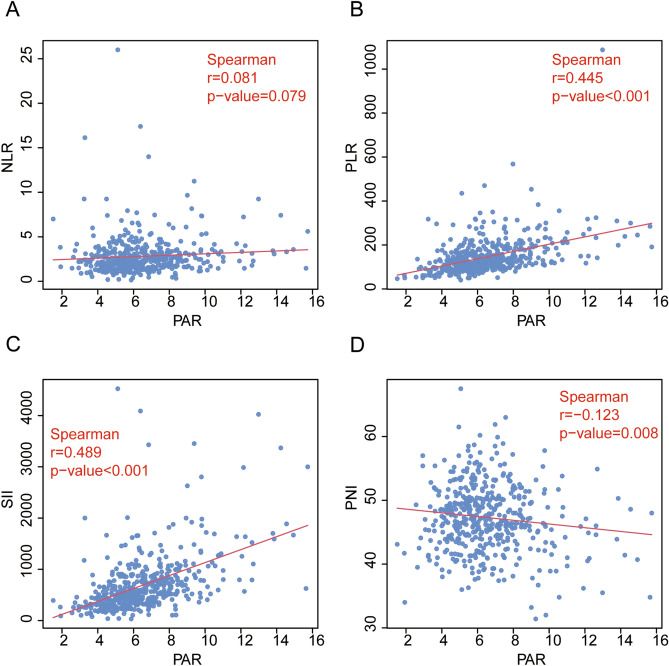
The association between the PAR and the patient clinicopathological variables of this study are shown in Table 1. We found that high PAR level was significantly associated with male (p = 0.007), age ≤ 70 years old (p = 0.020), deeper tumor invasion (T stage, p = 0.003), more advanced TNM stage (p = 0.001), Chemotherapy (p = 0.031), higher PLR (p < 0.001) and higher SII (p < 0.001). In additional, Spearman analyses showed a positive correlation between PAR and PLR (r = 0.445, P < 0.001; Fig. 1B) and SII (r = 0.489, P < 0.001; Fig. 1C). The PAR was negatively correlated with the PNI (r = − 0.123, P = 0.008; Fig. 1D) and no significant correlation with NLR (r = 0.081, P = 0.079; Fig. 1A).
Figure. 1.
Correlations between PAR and (A) NLR, (B) PLR, (C) SII and (D) PNI were evaluated via Spearman’s correlation analysis in the whole patients.
The prognostic significance of NLR, PLR SII, PNI and PAR
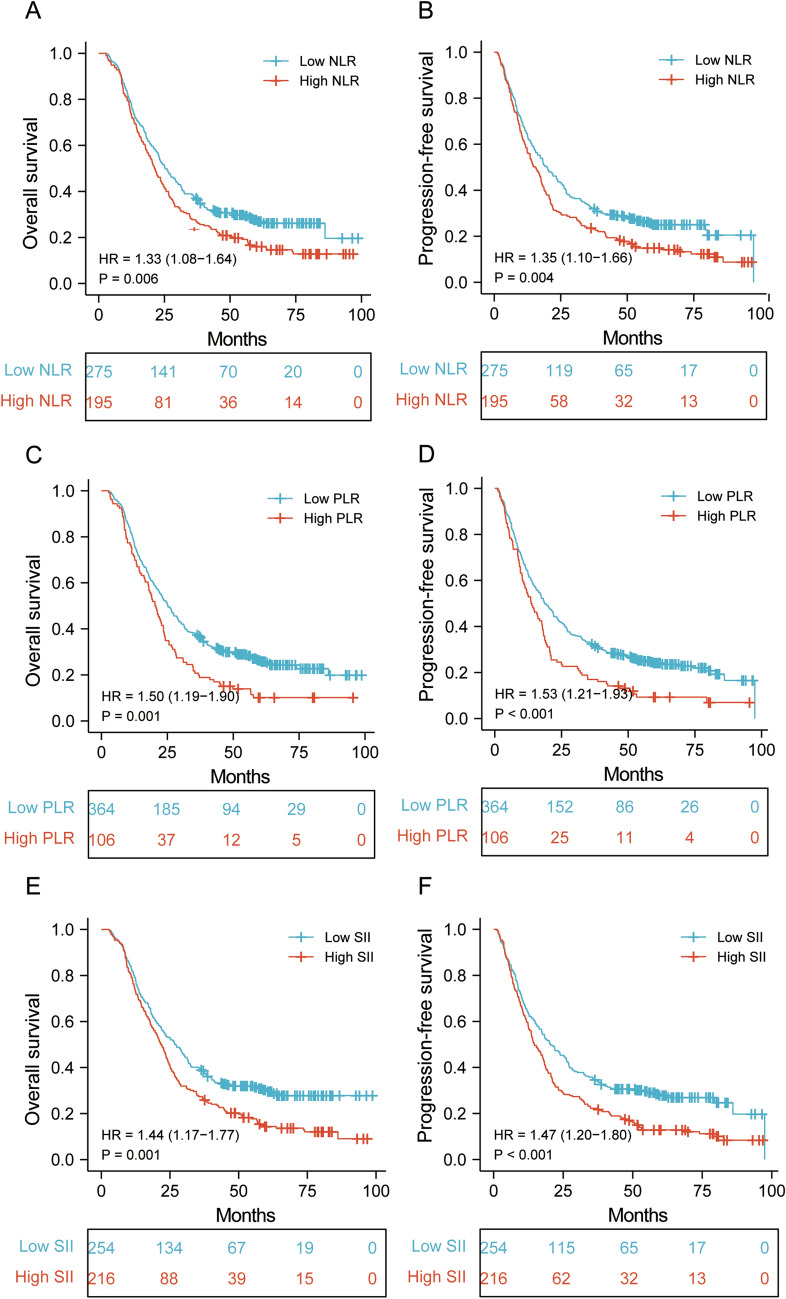
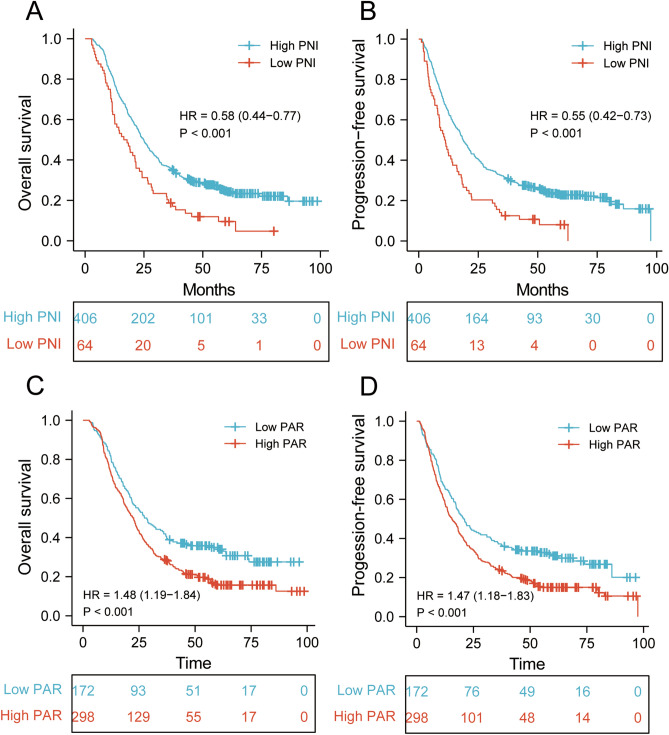
The median follow-up time was 23.5 months (range 2–98.7 months). The Kaplan–Meier survival analysis demonstrated that higher NLR, PLR, SII, and PAR were significantly correlated with shorter survival time (Figs. 2 and 3). The 1-, 3-, and 5-year PFS of ESCC patients with low and high PARs were 66.9%, 37.2%, 31.1%; and 59.7%, 24.2%, 15.0%, respectively (Fig. 3D). The low-PAR group had a significantly higher PFS rate than that of the high-PAR group (P < 0.001). The 1-, 3-, and 5-year OS rates were 74.5%, 28.9%, and 15.7%, respectively, in the high-PAR group and 83.1%, 42.4%, and 35.0%, respectively, in the low-PAR group (Fig. 3C).
Figure. 2.
Survival outcomes in ESCC patients undergoing definitive radiotherapy stratified by NLR, PLR and SII. (A) Overall survival and (B) progression-free survival between low and high NLR groups; (C) Overall survival and (D) progression-free survival between low and high PLR groups. (E) Overall survival and (F) progression-free survival between low and high SII groups.
Figure. 3.
Survival outcomes in ESCC patients undergoing definitive radiotherapy stratified by PNI, PAR. (A) Overall survival and (B) progression-free survival between low and high PNI groups; (C) Overall survival and (D) progression-free survival between low and high PAR groups.
Univariate Cox regression analysis demonstrated T stage, TNM stage, adjuvant chemotherapy, NLR, PLR, SII, PNI and PAR prognostic factors (P = 0.005, P < 0.001, P = 0.003, P = 0.004, P < 0.001, P < 0.001, P < 0.001, and P < 0.001, respectively for PFS; P = 0.005, P < 0.001, P = 0.007, P = 0.006, P < 0.001, P < 0.001, P < 0.001 and P < 0.001,respectively for OS) and as significant prognostic factor for both PFS and OS (Tables 2 and 3). All the eight clinicopathological characteristics were further investigated in the multivariate cox regression analysis. As shown in Table 2, TNM stage [hazard ratio (HR) 2.147, 95% confidence interval (CI): 1.376–3.351, P < 0.001], adjuvant chemotherapy (HR: 0.618, 95% CI: 0.493–0.774, P < 0.001), PNI (HR: 0.691, 95% CI: 0.505–0.944, P = 0.020) and PAR (HR: 1.298, 95% CI: 1.014–1.661, P = 0.038) were identified as independent prognostic factors in ESCC patient for predicting PFS. And in Table 3, TNM stage (HR: 2.147, 95% CI: 1.350–3.414, P = 0.001), adjuvant chemotherapy (HR: 0.647, 95% CI: 0.515–0.813, P < 0.001) and PAR (HR: 1.312, 95% CI: 1.021–1.685, P = 0.033) were identified as independent prognostic factors in ESCC patient for predicting OS.
Table 2.
Univariable and multivariable Cox regression analysis for progression-free survival.
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Gender | ||||
| Male/Female | 0.983 (0.784–1.232) | 0.879 | ||
| Age | ||||
| ≤ 70/ > 70 | 1.046 (0.846–1.292) | 0.679 | ||
| Location | ||||
| Cervical + Upper/Middle + Lower | 1.155 (0.926–1.440) | 0.202 | ||
| T stage | ||||
| T2-3/T4 | 1.335 (1.089–1.636) | 0.005 | 0.683 (0.439–1.063) | 0.091 |
| N stage | ||||
| N0/N + | 1.240 (0.973–1.581) | 0.083 | ||
| cTNM stage | ||||
| II + III/IV | 1.458 (1.188–1.788) | < 0.001 | 2.147 (1.376–3.351) | < 0.001 |
| RT technique | ||||
| 2D-CRT/IMRT + 3D-CRT | 0.877 (0.593–1.298) | 0.512 | ||
| Adjuvant chemotherapy | ||||
| No/Yes | 0.726 (0.586–0.898) | 0.003 | 0.618 (0.493–0.774) | < 0.001 |
| Marrow depression | ||||
| No/Yes | 0.820 (0.670–1.005) | 0.055 | ||
| NLR | ||||
| < 2.62/ ≥ 2.62 | 1.352 (1.103–1.658) | 0.004 | 1.103 (0.823–1.479) | 0.513 |
| PLR | ||||
| < 180/ ≥ 180 | 1.529 (1.211–1.930) | < 0.001 | 1.109 (0.833–1.476) | 0.478 |
| SII | ||||
| < 577.7/ ≥ 577.7 | 1.466 (1.196–1.796) | < 0.001 | 1.209 (0.886–1.649) | 0.232 |
| PNI | ||||
| < 41.5/ ≥ 41.5 | 0.554 (0.419–0.733) | < 0.001 | 0.691 (0.505–0.944) | 0.020 |
| PAR | ||||
| < 5.7 × 109/ ≥ 5.7 × 109 | 1.470 (1.183–1.827) | < 0.001 | 1.298 (1.014–1.661) | 0.038 |
HR hazard ratio, CI confidence interval.
Table 3.
Univariable and multivariable Cox regression analysis for overall survival.
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Gender | ||||
| Male/Female | 0.994 (0.790–1.250) | 0.960 | ||
| Age | ||||
| ≤ 70/ > 70 | 1.008 (0.812–1.251) | 0.944 | ||
| Location | ||||
| Cervical + Upper/Middle + Lower | 1.086 (0.870–1.356) | 0.468 | ||
| T stage | ||||
| T2-3/T4 | 1.341 (1.091–1.648) | 0.005 | 0.667 (0.421–1.057) | 0.085 |
| N stage | ||||
| N0/N + | 1.223 (0.957–1.562) | 0.107 | ||
| cTNM stage | ||||
| II + III/IV | 1.461 (1.188–1.798) | < 0.001 | 2.147 (1.350–3.414) | 0.001 |
| RT technique | ||||
| 2D-CRT/IMRT + 3D-CRT | 0.769 (0.516–1.147) | 0.199 | ||
| Adjuvant chemotherapy | ||||
| No/Yes | 0.742 (0.597–0.923) | 0.007 | 0.647 (0.515–0.813) | < 0.001 |
| Marrow depression | ||||
| No/Yes | 0.861 (0.701–1.057) | 0.152 | ||
| NLR | ||||
| < 2.62/ ≥ 2.62 | 1.333 (1.085–1.639) | 0.006 | 1.108 (0.820–1.496) | 0.505 |
| PLR | ||||
| < 180/ ≥ 180 | 1.503 (1.187–1.902) | < 0.001 | 1.095 (0.817–1.467) | 0.545 |
| SII | ||||
| < 577.7/ ≥ 577.7 | 1.440 (1.172–1.769) | < 0.001 | 1.170 (0.852–1.606) | 0.333 |
| PNI | ||||
| < 41.5/ ≥ 41.5 | 0.581 (0.438–0.770) | < 0.001 | 0.732 (0.532–1.007) | 0.055 |
| PAR | ||||
| < 5.7 × 109/ ≥ 5.7 × 109 | 1.480 (1.187–1.845) | < 0.001 | 1.312 (1.021–1.685) | 0.033 |
HR hazard ratio, CI confidence interval.
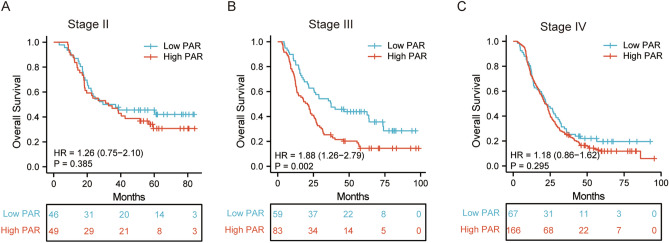
Furthermore, we explored the prognostic value of PAR in a subgroup analysis which was stratified by AJCC TNM stage. The result showed the high-PAR was significant shorter OS in stages III (P = 0.002), Fig. 4B). Although there was no statistical significance in the subgroup analyses of OS in stages II (P = 0.385, Fig. 4A) and stage IV (P = 0.295, Fig. 4C), the trend of worse prognosis in high-PAR patients was consistent.
Figure. 4.
Survival outcomes in ESCC patients undergoing definitive radiotherapy stratified by PAR in stage II (A), stage III (B), and stage IV (C) subgroup.
Discussion
Inflammation in the microenvironment of tumors is known to promote both carcinogenesis and disease progression and could reduce the efficacy of systemic treatments21. Platelet, as a critical component in hemostasis, is also observed to be associated with systemic inflammation and the immune system22,23. Nutritional status is also a critical part of cancer management as malnutrition is frequent in cancer patients and is correlated with poor prognosis24–26. Previous studies have demonstrated albumin-related malnutrition was significantly associated with poor prognosis in ESCC patients27,28. Hence, a novel inflammation-based indicator PAR was established, representing both inflammation and nutritional status.
The current study first evaluated the prognostic value of a pretreatment PAR in ESCC patients who received definitive radiotherapy ± adjuvant chemotherapy. Our results showed that patients with a low pretreatment PAR(< 5.7 × 109) had a significantly better prognosis in both PFS and OS than those with a high pretreatment PAR(≥ 5.7 × 109). A high PAR was more likely to associate with an younger age, a more advanced TNM stage, suggesting that PAR could reflect tumor progression in patients with ESCC. Multivariable analyses also validated the PAR as an independent indicator of PFS and OS. Taken together, our results demonstrated that pretreatment PAR is an independent prognostic factor for patients with ESCC who received definitive radiotherapy.
Comparing to PAR, the existing inflammation-based indicators, including NLR, PLR, SII and PNI, showed no statistical significance as an independent prognostic factor in the multivariable cox regression analysis. Previous studies showed that high pretreatment of NLR and PLR were independent prognostic markers for OS in esophageal cancer patients. In the current study, NLR and PLR showed a correlation with OS but failed to maintain significance in multivariable cox analysis. The findings were consistent with Geng et al. research29. Likewise, SII also demonstrated no independent prognostic value in this study.
It’s been known that inflammation not only participates in tumorigenesis, malignant progression, and metastasis but also interplay with antitumor immunity30. A number of studies have revealed that high platelet counts can affect tumor development and result in thrombocytosis, which is an unfavorable factor for patients’ clinical outcomes31. It has been hypothesized that platelet was activated by cytokines secreted by tumor cells, such as platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), transforming growth factor-β1 (TGF-β1). Those cytokines are components of platelet and play a critical role in tumor development, including tumor proliferation, angiogenesis, and metastasis32. Moreover, platelet could shield peripheral circulating tumor cells and interfere with natural killer cells for recognition of tumor cells, which enhanced their metastatic potential33. Most recently, more emphasis has been put on the platelets and their complicated interplay with leukocytes and tumor cells in the tumor microenvironment, which could help us to better understand the mechanism behind the commonly used antiplatelet therapy and shed light on novel cancer therapy 34.
Low serum albumin concentration could also lead to a high PAR. Previous studies have demonstrated that pretreatment serum albumin is associated with short life expectancy in cancer patients35. Malnutrition is a significant problem in digestive cancer patients, especially in those with locally advanced esophageal cancer undergoing definitive radiotherapy. The tumor-caused restriction of oral intake and radiation-induced esophagitis are the main reasons that account for undernutrition. Patients with an undernutrition status before treatment were demonstrated to be associated with poor treatment response and prognosis36. In our study, we found that patients with high serum albumin concentration had a better survival outcome than those with low serum albumin concentration (as shown in Supplementary Fig. 3), which is consistent with previous findings. Moreover, albumin synthesis was suppressed by the systemic inflammation. Consequentially the immune system functions were impaired due to hypoalbuminemia, and tumor cells could progress more easily due to immune suppression. Therefore, risk stratification based on inflammation-nutritional indicators is of great significance and will help the clinical physician to provide timely and effective nutritional intervention.
Although our study demonstrated the prognostic significance of PAR in patients with ESCC, several limitations in our study still need to be noted. First, this is a retrospective study from a single center, which may lead to selection bias. Second, platelet counts and serum albumin levels could be influenced by other factors such as coagulation disorder and liver dysfunction, which might affect the predictive accuracy of prognosis. Third, the optimal cut-off point of PAR might fluctuate as the evaluated population changed. Hence, further larger-scale prospective studies are needed to confirm the preliminary results of our study.
Conclusion
Our study demonstrated that the PAR, a novel independent risk predictor, had the potential for predicting prognosis of ESCC patients undergoing definitive radiotherapy. Measurement of the PAR is convenient, inexpensive, and reliable in the routine clinical practice. Anti-inflammation therapy and/or nutritional interventions should be considered for patients with low pretreatment PAR levels. Therefore, PAR measurement will help the clinical decision-making according to the individual difference. Future validation from prospective larger-scale studies is warranted.
Supplementary Information
Acknowledgements
We would like to thank all the patients and working staff in the Fujian Medical University Cancer Hospital for their support.
Abbreviations
- PAR
Platelet to albumin ratio
- ESCC
Esophageal squamous cell carcinoma
- PFS
Progression-free survival
- OS
Overall survival
- EC
Esophageal cancer
- PNI
Prognostic nutritional index
- 2DRT
Two-dimensional conformal radiation therapy
- 3DRT
Three-dimensional conformal radiation therapy
- IMRT
Intensity-modulated radiation therapy
- NLR
Neutrophil to lymphocyte ratio
- PLR
Platelet to lymphocyte ratio
- SII
Systemic immune-inflammation index
- GTV
Gross tumor volume
- CTV
Clinical target volume
- PTV
Planning target volume
Author contributions
Z.H., Q.Z., J.L. conceived the study design. Q.Z., Y.W., T.L., H.Z. participated in data acquisition and interpretation. Z.H., M.Z., L.L., H.L. analyzed the data. Z.H., Q.Z., Y.Y. wrote the manuscript. Z.W., J.L. revised the article. All authors read and approved the final manuscript.
Funding
The project was funded by the grants from Innovation of Science and Technology, Fujian Province (Grant Number: 2018Y9111) and the Financial Foundation of Fujian Province (Grant Number: (2019)827).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Zhiyu Huang and Qunhao Zheng.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-07546-0.
References
- 1.Global Burden of Disease Cancer C. Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, Abdel-Rahman O, Abdelalim A, Abdoli A, Abdollahpour I, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990–2017: A systematic analysis for the global burden of disease study. JAMA Oncol. 2019;5(12):1749–1768. doi: 10.1001/jamaoncol.2019.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.van Rossum PSN, Mohammad NH, Vleggaar FP, van Hillegersberg R. Treatment for unresectable or metastatic oesophageal cancer: Current evidence and trends. Nat. Rev. Gastroenterol. Hepatol. 2018;15(4):235–249. doi: 10.1038/nrgastro.2017.162. [DOI] [PubMed] [Google Scholar]
- 4.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493–e503. doi: 10.1016/S1470-2045(14)70263-3. [DOI] [PubMed] [Google Scholar]
- 6.Zhou XL, Li YQ, Zhu WG, Yu CH, Song YQ, Wang WW, He DC, Tao GZ, Tong YS. Neutrophil-to-lymphocyte ratio as a prognostic biomarker for patients with locally advanced esophageal squamous cell carcinoma treated with definitive chemoradiotherapy. Sci. Rep. 2017;7:42581. doi: 10.1038/srep42581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu G, Yao Y, Bai C, Zeng J, Shi D, Gu X, Shi X, Song Y. Combination of platelet to lymphocyte ratio and neutrophil to lymphocyte ratio is a useful prognostic factor in advanced non-small cell lung cancer patients. Thorac. Cancer. 2015;6(3):275–287. doi: 10.1111/1759-7714.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim MR, Kim AS, Choi HI, Jung JH, Park JY, Ko HJ. Inflammatory markers for predicting overall survival in gastric cancer patients: A systematic review and meta-analysis. PLoS One. 2020;15(7):e0236445. doi: 10.1371/journal.pone.0236445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akgul O, Cetinkaya E, Yalaza M, Ozden S, Tez M. Prognostic efficacy of inflammation-based markers in patients with curative colorectal cancer resection. World J. Gastrointest. Oncol. 2017;9(7):300–307. doi: 10.4251/wjgo.v9.i7.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng H, Luo X. Prognostic significance of elevated pretreatment systemic inflammatory markers for patients with prostate cancer: A meta-analysis. Cancer Cell Int. 2019;19:70. doi: 10.1186/s12935-019-0785-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sapienza C, Issa JP. Diet, nutrition, and cancer epigenetics. Annu. Rev. Nutr. 2016;36:665–681. doi: 10.1146/annurev-nutr-121415-112634. [DOI] [PubMed] [Google Scholar]
- 12.Sakurai K, Ohira M, Tamura T, Toyokawa T, Amano R, Kubo N, Tanaka H, Muguruma K, Yashiro M, Maeda K, et al. Predictive potential of preoperative nutritional status in long-term outcome projections for patients with gastric cancer. Ann. Surg. Oncol. 2016;23(2):525–533. doi: 10.1245/s10434-015-4814-7. [DOI] [PubMed] [Google Scholar]
- 13.Toyokawa T, Kubo N, Tamura T, Sakurai K, Amano R, Tanaka H, Muguruma K, Yashiro M, Hirakawa K, Ohira M. The pretreatment controlling nutritional status (CONUT) score is an independent prognostic factor in patients with resectable thoracic esophageal squamous cell carcinoma: Results from a retrospective study. BMC Cancer. 2016;16:722. doi: 10.1186/s12885-016-2696-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo M, Sun T, Zhao Z, Ming L. Preoperative platelet to albumin ratio predicts outcome of patients with non-small-cell lung cancer. Ann. Thorac. Cardiovasc. Surg. 2021;27(2):84–90. doi: 10.5761/atcs.oa.20-00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li C, Peng W, Zhang XY, Wen TF, Chen LP. The preoperative platelet to albumin ratio predicts the prognosis of hepatocellular carcinoma patients without portal hypertension after liver resection. Medicine (Baltimore) 2019;98(45):e17920. doi: 10.1097/MD.0000000000017920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shirai Y, Shiba H, Haruki K, Horiuchi T, Saito N, Fujiwara Y, Sakamoto T, Uwagawa T, Yanaga K. Preoperative platelet-to-albumin ratio predicts prognosis of patients with pancreatic ductal adenocarcinoma after pancreatic resection. Anticancer Res. 2017;37(2):787–793. doi: 10.21873/anticanres.11378. [DOI] [PubMed] [Google Scholar]
- 17.Saito N, Shirai Y, Horiuchi T, Sugano H, Shiba H, Sakamoto T, Uwagawa T, Yanaga K. Preoperative platelet to albumin ratio predicts outcome of patients with cholangiocarcinoma. Anticancer Res. 2018;38(2):987–992. doi: 10.21873/anticanres.12313. [DOI] [PubMed] [Google Scholar]
- 18.Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: Application to clinical practice. Ann. Cardiothorac. Surg. 2017;6(2):119–130. doi: 10.21037/acs.2017.03.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: A new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin. Cancer Res. 2004;10(21):7252–7259. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
- 20.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 21.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis. 2009;30(7):1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 22.Thomas MR, Storey RF. The role of platelets in inflammation. Thromb. Haemost. 2015;114(3):449–458. doi: 10.1160/TH14-12-1067. [DOI] [PubMed] [Google Scholar]
- 23.Kral JB, Schrottmaier WC, Salzmann M, Assinger A. Platelet interaction with innate immune cells. Transfus. Med. Hemother. 2016;43(2):78–88. doi: 10.1159/000444807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pressoir M, Desne S, Berchery D, Rossignol G, Poiree B, Meslier M, Traversier S, Vittot M, Simon M, Gekiere JP, et al. Prevalence, risk factors and clinical implications of malnutrition in French comprehensive cancer centres. Br. J. Cancer. 2010;102(6):966–971. doi: 10.1038/sj.bjc.6605578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alvaro Sanz E, Garrido Siles M, Rey Fernandez L, Villatoro Roldan R, Rueda Dominguez A, Abiles J. Nutritional risk and malnutrition rates at diagnosis of cancer in patients treated in outpatient settings: Early intervention protocol. Nutrition. 2019;57:148–153. doi: 10.1016/j.nut.2018.05.021. [DOI] [PubMed] [Google Scholar]
- 26.Arends J. Struggling with nutrition in patients with advanced cancer: nutrition and nourishment: Focusing on metabolism and supportive care. Ann. Oncol. 2018;29:ii27–ii34. doi: 10.1093/annonc/mdy093. [DOI] [PubMed] [Google Scholar]
- 27.Lindenmann J, Fink-Neuboeck N, Koesslbacher M, Pichler M, Stojakovic T, Roller RE, Maier A, Anegg U, Smolle J, Smolle-Juettner FM. The influence of elevated levels of C-reactive protein and hypoalbuminemia on survival in patients with advanced inoperable esophageal cancer undergoing palliative treatment. J. Surg. Oncol. 2014;110(6):645–650. doi: 10.1002/jso.23711. [DOI] [PubMed] [Google Scholar]
- 28.Sun SY, Chen PP, Meng LX, Li L, Mo ZX, Sun CH, Wang Y, Liang FH. High preoperative plasma fibrinogen and serum albumin score is associated with poor survival in operable esophageal squamous cell carcinoma. Dis. Esophagus. 2019;32(1):doy057. doi: 10.1093/dote/doy057. [DOI] [PubMed] [Google Scholar]
- 29.Geng Y, Shao Y, Zhu D, Zheng X, Zhou Q, Zhou W, Ni X, Wu C, Jiang J. Systemic immune-inflammation index predicts prognosis of patients with esophageal squamous cell carcinoma: A propensity score-matched analysis. Sci. Rep. 2016;6:39482. doi: 10.1038/srep39482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dougan M, Dranoff G. Immune therapy for cancer. Annu. Rev. Immunol. 2009;27:83–117. doi: 10.1146/annurev.immunol.021908.132544. [DOI] [PubMed] [Google Scholar]
- 31.Horsted F, West J, Grainge MJ. Risk of venous thromboembolism in patients with cancer: A systematic review and meta-analysis. PLoS Med. 2012;9(7):e1001275. doi: 10.1371/journal.pmed.1001275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qi C, Li B, Guo S, Wei B, Shao C, Li J, Yang Y, Zhang Q, Li J, He X, et al. P-selectin-mediated adhesion between platelets and tumor cells promotes intestinal tumorigenesis in apc(Min/+) Mice. Int. J. Biol. Sci. 2015;11(6):679–687. doi: 10.7150/ijbs.11589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, Jirouskova M, Degen JL. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005;105(1):178–185. doi: 10.1182/blood-2004-06-2272. [DOI] [PubMed] [Google Scholar]
- 34.Stoiber D, Assinger A. Platelet-leukocyte interplay in cancer development and progression. Cells. 2020;9(4):855. doi: 10.3390/cells9040855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: A systematic review of the epidemiological literature. Nutr. J. 2010;9:69. doi: 10.1186/1475-2891-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cox S, Powell C, Carter B, Hurt C, Mukherjee S, Crosby TD. Role of nutritional status and intervention in oesophageal cancer treated with definitive chemoradiotherapy: Outcomes from SCOPE1. Br. J. Cancer. 2016;115(2):172–177. doi: 10.1038/bjc.2016.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.