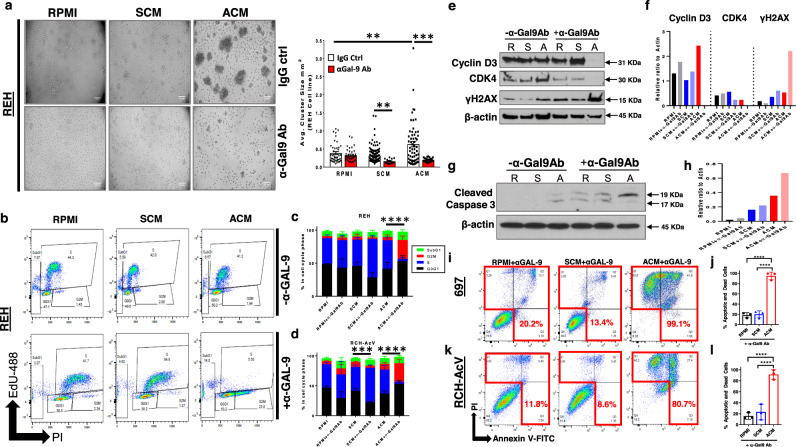
Fig. 6. Treatment with αGalectin-9 antibody alters cell cycle progression and induces cell death in human B-ALL cells in the presence of adipocyte-secreted factors.
a Human B-ALL cell line REH was cultured for 24 h in unconditioned medium (RPMI), SCM, or ACM and then treated with αGalectin-9 (αGAL-9) or IgG control (IgG ctrl) antibody for an additional 2 days. On day 3 of culture, brightfield images were obtained and cell cluster sizes were quantified. b–d, REH cells were treated with IgG (no αGAL-9 antibody) or αGAL-9 antibody for 24 h. After a day of treatment, 5-ethynyl-2’-deoxyuridine (EdU) was added to cultures for 2 h and then cells were permeabilized and stained with propidium iodide (PI). The fraction of cells in S (EdU + ), G1 (2 N DNA content), and G2/M (4 N DNA content) phases of the cell cycle was determined by flow cytometric analysis. Representative data are shown in b. e-h, Lysates were prepared after 48 h of culture in RPMI (R), SCM (S), or ACM (A) with IgG (no αGAL-9 antibody) or αGAL-9 antibody. The presence of Cyclin D3, CDK4, γH2AX, and β-actin (loading control) protein was determined by western blot analysis. Primary data is shown in e and g and quantitative data is shown in f and h. i–l, Human B-ALL cell lines (697 and RCH-AcV) were cultured for 24 h in RPMI, SCM, or ACM and then treated with anti-GAL-9 antibody (αGAL-9 Ab) or an IgG control antibody (IgG ctrl) for an additional 2 days. The percentage of apoptotic and dead cells on day 3 of culture was determined using Annexin-V/ PI staining followed by flow cytometric analysis. Representative data are shown in i,k. Means ± s.d. are shown (*p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001, n = 4 independent experiments, one-way ANOVA with Tukey’s post-test). In b–d ****p < 0.0001, n = 3 independent experiments, two-sided Student’s t-test of the G2/M cell cycle profiles. Western blot source data are provided in the Source Data file.