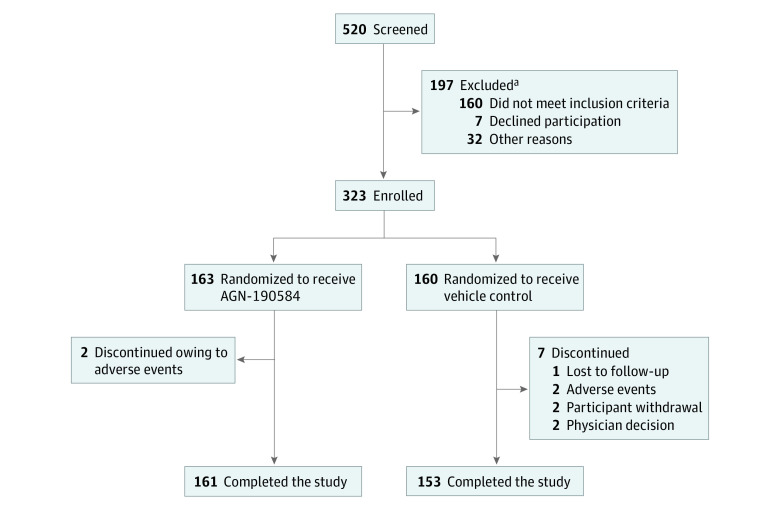
Figure 1. Participant Disposition.
The adverse events leading to study discontinuation were bradycardia (n = 1) and dyschromatopsia and bilateral visual field defect (n = 1) in the AGN-190584 group and corneal abrasion (n = 1) and headache and migraine (n = 1) in the vehicle group.
aParticipants could fail screening owing to multiple reasons.