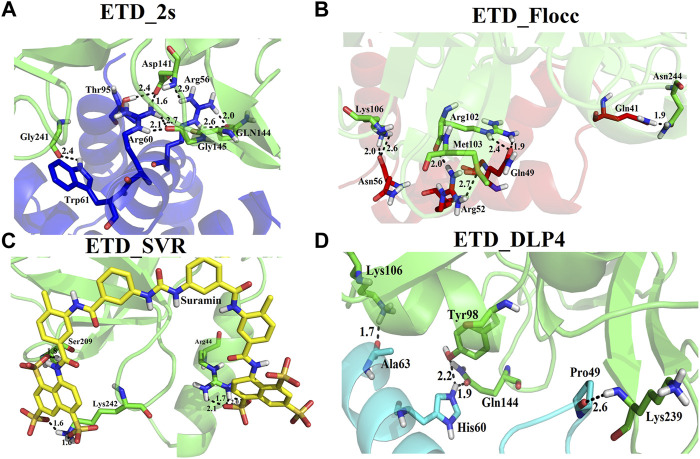
FIGURE 8.
Proposed mechanisms for ETs. (A) The catalytic triad (His72, Asp120, and Ser195) and the amino acid residues Pro192, Gly193 (red sticks), and hydrolytic water (green sphere); the enzyme is inactive in this state. (B) The enzyme (red) becomes active after binding to the substrate (green), by disrupting the bond with Pro192. (C) The enzyme attacks the substrate (peptide) (green sticks) carboxyl group. (C) Tetrahedral intermediate. (D) Donation of H+ by His72 to peptide for leaving group, and breakage of the tetrahedral intermediate and release of the first product. (E) Splitting of the hydrolytic water molecule into H+ and OH−. (F) The OH− attacks the bond between Ser195 and the peptide C-terminal end and liberates this part from the enzyme. (G) ET (red) and enzyme (green) complex.