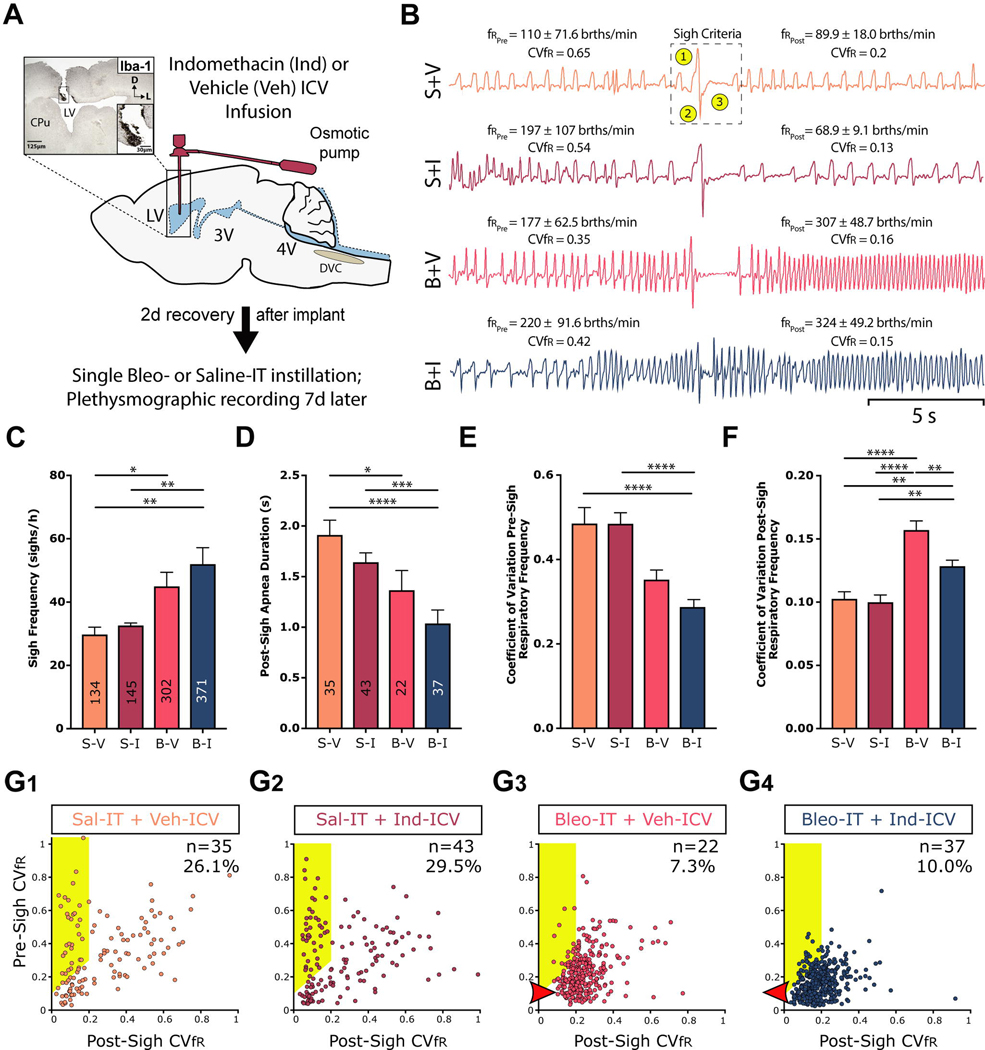
Figure 5:
SLI increases sigh frequency and modifies sigh dynamics, which is partially restored by ICV Indomethacin treatment.
A. Protocol. Rats received stereotaxic surgery to implant ICV cannulas attached to subcutaneously implanted mini-osmotic pumps (0.5 μl/h, 200 μl reservoir), which contained either vehicle (Veh, 50% DMSO) or Indomethacin (Ind, 8.3 μg/μl). 2d following cannulation, rats received Bleomycin (Bleo) or saline (Sal) intratracheally (IT); 7d later ventilatory pattern was recorded via whole body plethysmography and rats were euthanized for IHC experiments. Group data are from 7 rats in each group.
B. Representative sighs for each group. The mean ± standard deviation of: i) the respiratory frequency for the 10s before and after the sigh is included for each sigh, and the coefficient of variation for the respiratory frequency (CVfR). In the top tracing, we identified sighs based on three (highlighted) criteria: 1) an augmented inspiration, 2) augmented initial expiration and 3) a prolonged expiration, which is referred to as a post-sigh apnea.
C. The frequency of sighs was significantly greater in Bleo-IT treated rats that received Veh-ICV (B+V) when compared to saline-IT treated that received Veh- or Ind- ICV (S+V or S+I), and was not significantly different compared to Bleo-IT treated rats that received Ind-ICV (B+I) (B+V vs. S+V: P = 0.03; B+V vs. S+I: P = 0.104; B+V vs. B+I, P = 0.532). The frequency of sighs was also significantly greater in B+I treated rats compared to either of the control groups (B+I vs. S+I: P = 0.005; B+I vs. S+V: P = 0.0013; Kruskal-Wallis test with Dunn’s post hoc).
D. The duration of the apnea that followed a ‘resetting’ sigh was shorter in injured rats compared to sham rats, but Ind-ICV treatment did not alter apnea duration in Bleo- or saline-treated rats ((Figure 5D) B+V vs. S+V: P = 0.04; B+I vs. S+I: P = 0.0008; B+I vs. B+V: P = 0.89; S+V vs. S+I: P > 0.999; Kruskal-Wallis test with Dunn’s post-hoc).
E. The CVfR before a resetting sigh was not significantly different in B+V treated rats compared to either of the saline-IT control groups, or Bleo-IT rats that received Ind-ICV (B+V vs. S+V: P = 0.27; B+V vs. S+I: P = 0.06; B+V vs. B+I: P = 0.29). However, the pre-sigh CVfR for B+I rats compared to either of the control groups (B+I vs. S+I: P < 0.0001; B+I vs. S+V: P < 0.0001; Kruskal-Wallis test with Dunn’s post-hoc).
F. The CVfR after a resetting sigh was significantly increased in B+V treated rats compared to control groups and was significantly reduced in B+I (B+V vs. S+V: P< 0.0001; B+V: vs. S+I: P < 0.0001; B+V vs. B+I: P = 0.007). However, Ind-ICV treatment did not fully restore post-sigh CVfR in SLI rats; the CVfR of B+I rats was significantly greater compared to either of the control groups (B+I vs. S+I: P = 0.002; B+I vs. S+V: P = 0.005; Kruskal-Wallis test with Dunn’s post-hoc).
G. Plots of pre-sigh CVfR vs. post-sigh CVfR for G1) S+V, G2) S+I, G3) B+V, G4) B+I treated rats. ‘resetting’ sigh was bounded by definition at 0.2. Both sham (S+V and S+I) groups and the injured group treated with Ind (B+I) had less variability in fR than the B+V group. Thus, the ‘resetting’ sighs were more effective in the B+I than the B+V group.