Figure 1.
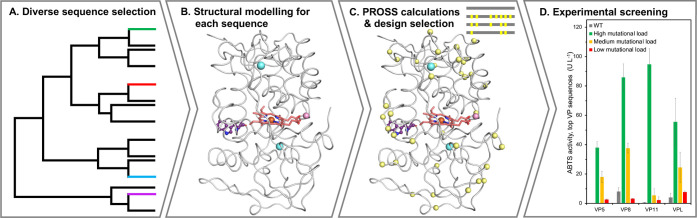
Key steps in the design of a diverse set of VPs. (A) VP sequences were collected from different databases, a phylogenetic tree was computed and 12 representative sequences were selected. (B) Selected sequences were modeled by trRosetta.25,26 For visualization, heme (red), manganese (pink), and calcium ions (blue) were superimposed from the VPL structure (PDB entry: 3FJW). The surface-reactive tryptophan is presented in purple balls-and-sticks. (C) PROSS stability-design calculations18,19 suggested dozens of mutations (yellow spheres). For each sequence, three designs with different mutational loads were selected for further experimental examination. (D) In an activity screen of proteins heterologously produced in yeast, the wildtype proteins show negligible functional expression, while the designs with the highest mutational load are highly active on the peroxidase substrate ABTS.