Fig. 1.
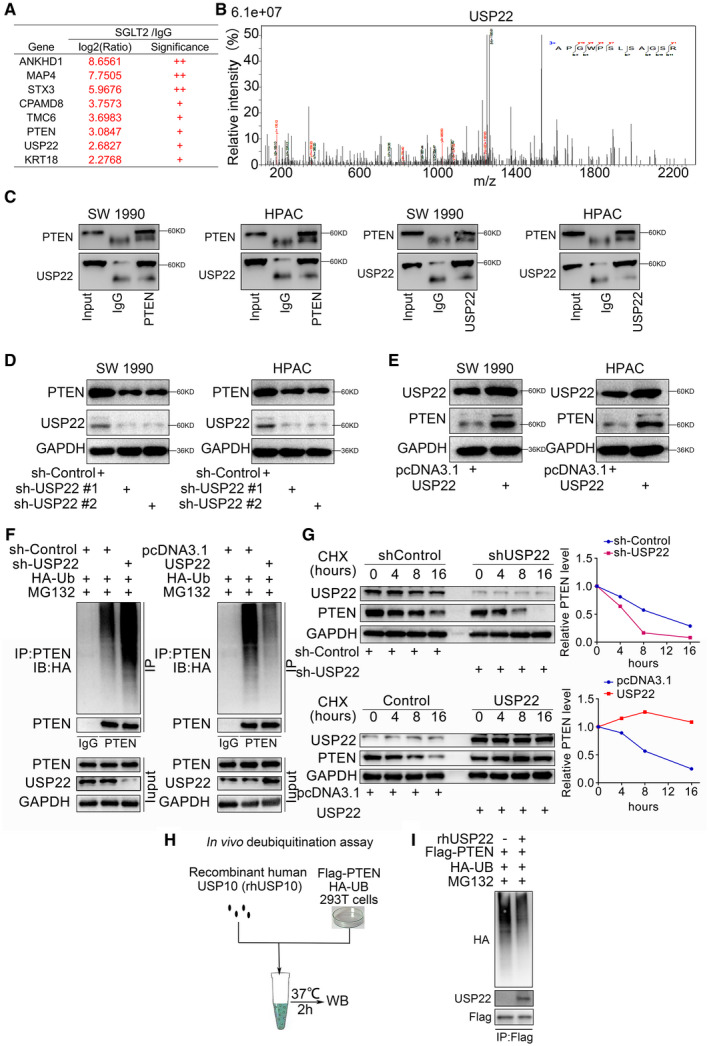
USP22 deubiquitinated PTEN and inhibited the degradation of PTEN in pancreatic cancer. (A,B) LC‐MS/MS identified an interaction between PTEN and USP22 by detecting a peptide of USP22 (n = 1 for IgG and PTEN group). (C) Coimmunoprecipitation showed the interaction between PTEN and USP22, which repeated for three replicates. (D,E) Western blot analysis of PTEN expression in SW 1990 and HPAC cells. GAPDH served as an internal reference and repeated for three replicates. (F) Western blot analysis in SW 1990 cells transfected with sh‐USP22 or USP22 plasmid for 48 h for western blot. Cells were treated with MG132 (10 µm) for 8 h before harvested. GAPDH served as an internal reference and repeated for three replicates. (G) Western Blot to show the PTEN expression in SW 1990 and HPAC cells. The cells were treated with cycloheximide for different duration. GAPDH served as an internal reference and repeated for three replicates. (H,I) Flag‐tagged PTEN and HA‐tagged UB plasmids were transfected into 293T cells. Subsequently, polyubiquitinated PTEN from the cell lysate pulled down by anti‐Flag IP resin and incubated with rhUSP22 protein for 2 h at 37 °C in vitro (H). Lysates were immunoblotted with indicated antibodies (I). GAPDH served as an internal reference and repeated for three replicates. GAPDH: Glyceraldehyde‐3‐phosphate dehydrogenase; IP: Immunoprecipitation.
