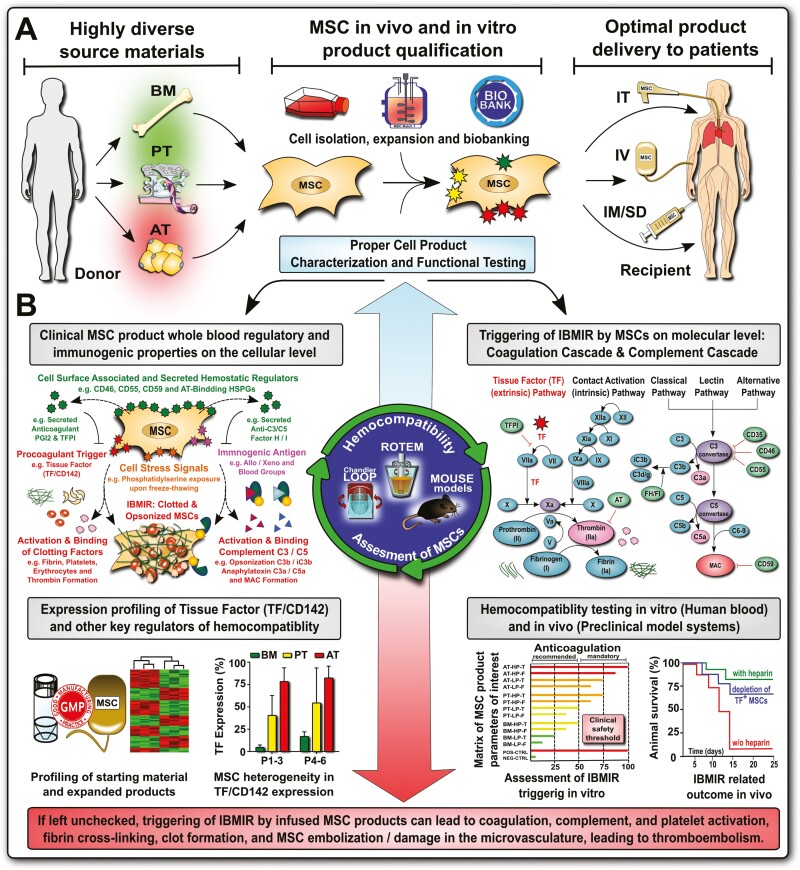
Figure 2.
Increasing mesenchymal stromal/stem cell (MSC) product diversification necessitates TF/CD142 screening and hemocompatibility assessment for intravascular MSC applications. (A) MSC products have greatly diversified in the past decade, for example, the tissue source that they are derived from, with bone marrow (BM), perinatal tissue (PT), and adipose tissue (AT) being the most frequent sources, which is decisive for the optimal mode of clinical delivery to patients, for example, intravascular (IV) infusion versus intramuscular or subdermal (IM/SD) injection, or intratracheal (IT) delivery12,18; and (B) Product qualification has shown large differences in expression of procoagulant tissue factor (TF/CD142) between different products (BM lowest, PT intermediate, and AT highest),12 which impacts on the cells’ hemocompatibility properties in vitro and in vivo, and may lead to adverse thrombotic reactions if left unchecked before clinical application. Abbreviations: complement factors C3, C5, C3b, iC3b, C3a, and C5a, and regulatory molecules: membrane cofactor protein (MCP/CD46), decay-accelerating factor (DAF/CD55), protectin (CD59), and factor H and I; coagulation factors I-XII (FI-XII), including their activated intermediates (eg, FXIIa) and regulators antithrombin (AT), tissue factor pathway inhibitor (TFPI) and prostacyclin (PGI2), and instant blood-mediated inflammatory reaction (IBMIR).