Graphical Abstract.
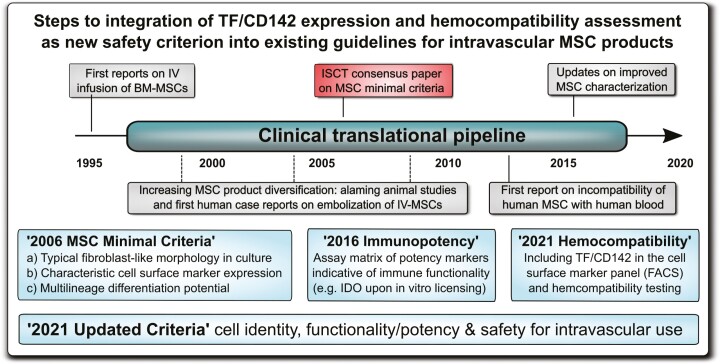
A broad spectrum of oversight impacts on MSC product safety in patients. We here outline the necessary steps toward integration of highly procoagulant tissue factor (TF/CD142) and hemocompatibility assessment of diversified intravascular MSC products as a new safety criterion into the existing MSC minimal criteria. Regulatory authorities and international societies should undertake coordinated efforts to update the already established guidelines.