Abstract
Purpose:
To assess the correlation between changes in hip capsule morphology with improvements in patient-reported outcome (PRO) scores after arthroscopic surgery for femoroacetabular impingement syndrome (FAIS) using the periportal capsulotomy technique.
Methods:
Twenty-eight patients with cam morphology FAIS (without arthritis, dysplasia, or hypermobility) were prospectively enrolled before arthroscopic labral repair and femoroplasty through periportal capsulotomy (anterolateral/midanterior portals) without closure. Patients completed the Hip Disability and Osteoarthritis Outcomes Score (HOOS) and had nonarthrographic 3T magnetic resonance imaging (MRI) scans of the affected hip before and 1 year after surgery. Anterior capsule thickness, posterior capsule thickness, anterior-posterior capsule thickness ratio, and proximal-distal anterior capsule thickness ratio were measured on axial-oblique MRI sequences. Pearson correlation coefficients were calculated to determine the association between hip capsule morphology and PRO scores.
Results:
Postoperative imaging showed that for all 28 patients (12 female), labral repairs and capsulotomies had healed within 1 year of surgery. Analysis revealed postoperative decreases in anterior hip capsule thickness (1395.4 ±508.4 mm3 vs 1758.4 ±487.9 mm3; P=.003) and anterior-posterior capsule thickness ratio (0.92± 0.33 vs 1.12±0.38; P = .02). Higher preoperative anterior-posterior capsule thickness ratio correlated with lower preoperative scores for HOOS pain (R=0.43; P = .02), activities of daily living (ADL) (R = −0.43; P = .02), and sport (R=−0.38; P = .04). Greater decrease from preoperative to postoperative anterior-posterior capsule thickness ratio correlated with greater improvement for HOOS pain(R = −0.40;P =.04),ADL(R = −0.45;P = .02),and sport(R = −0.46; P = .02).
Conclusions:
Periportal capsulotomy without closure demonstrates capsule healing by 1 year after arthroscopic FAIS treatment. Changes in hip capsule morphology including decreased anterior-posterior capsule thickness ratio after surgery may be correlated with improvements in patient pain, function, and ability to return to sports.
Level of Evidence:
Level II, prospective cohort study.
Femoroacetabular impingement syndrome (FAIS) is a condition in which abnormal morphologic features of the femoral head or acetabulum cause progressive damage to the labrum and cartilage through repetitive hip motion and has become an increasingly recognized cause of hip pain in the younger, more active population.1–4 If FAIS fails conservative management, surgical interventions are performed to correct the bony morphologic abnormalities and to treat the damaged labrum.3,5–7 Among these interventions, arthroscopic surgery has become commonly used in recent years, because it has been shown to significantly decrease pain and improve hip function in FAIS patients.8–12
Although there have been many studies demonstrating excellent patient-reported outcomes after arthroscopic procedures such as labral repairs and osteochondroplasty,13–15 there is a lack of consensus regarding proper capsular management during hip arthroscopy for FAIS. Although most surgeons use an interportal capsulotomy approach,16 periportal capsulotomy,17 and T-capsulotomy18 are 2 other techniques that are often used to access the hip joint during arthroscopic surgery. Additionally, there is ongoing debate on whether capsular repair should be regularly performed after capsulotomy, particularly with respect to the type of capsulotomy performed.17,19–21 For example, Chambers et al.17 demonstrated improved patient outcomes after periportal capsulotomy without closure because with this technique, incomplete transection of the iliofemoral ligament obviated the need for capsule closure.
Some studies have further explored the relationship between hip capsule anatomy and patient symptoms to better understand the optimal surgical approach to capsular management. Because magnetic resonance imaging (MRI) has been found to be useful in characterizing capsular integrity and thickness, the anatomy of the hip capsule has been shown to be related to FAIS pathophysiology and symptoms. One study showed that cam morphology hips have a thicker hip capsule compared to asymptomatic controls.22 Other studies have found that on MRI, a thicker anterior hip capsule is correlated with decreased hip range of motion in FAIS whereas a thinner anterior hip capsule is correlated with clinical laxity.15,23,24 Patient-reported outcomes (PRO) scores have also been shown to be associated with hip capsule characteristics, because a previous study found that an increased anterior to posterior hip capsule volume ratio in native FAIS hips correlated with worse patient-reported pain scores.25 However, less is known about how changes in hip capsule characteristics after arthroscopic surgery are correlated with changes in PRO scores for postoperative pain, function, and symptoms. Assessment of postoperative hip capsule morphology after arthroscopic treatment for FAIS using the periportal capsulotomy would help to better understand whether the capsule heals fully and the relationship between changes in hip capsule morphology after surgery and changes in PRO scores.
The purpose of this study is to assess the correlation between changes in hip capsule morphology with improvements in PRO scores after arthroscopic surgery for FAIS using the periportal capsulotomy technique. We hypothesize that a greater decrease from preoperative to postoperative anterior hip capsule thickness is associated with greater improvements in PRO scores.
Methods
Patient cohort
Study protocols and procedures underwent institutional review board approval. Twenty-eight patients (28 hips) with cam morphology and FAIS undergoing primary arthroscopic surgery for femoroplasty and labral repair were prospectively enrolled before surgery from a single tertiary-referral hospital’s hip preservation center. Written informed consent was obtained from each patient prior to study enrollment. Patient age, body mass index, and sex were recorded prior to surgery. Patient inclusion criteria included age between 18 and 50, body mass index < 30 kg/m2, no radiographic findings of joint space narrowing (Tönnis grade 0 or 1), no radiographic findings of pincer morphology (lateral center edge angle <40° and no crossover sign), no signs of joint hyperlaxity (Beighton score < 4), and diagnosis of cam morphology FAIS on physical and radiographic examination (alpha angle > 55° on Dunn lateral view) refractory to at least 6 weeks of conservative treatments such as activity modification, physical therapy, nonsteroidal anti-inflammatory drugs, or intra-articular corticosteroid injections. Patient exclusion criteria included hip dysplasia, borderline dysplasia, abnormal femoral or acetabular version, previous surgery, and femoral abnormalities such as Perthes disease and slipped capital femoral epiphysis. Only cam morphology FAIS patients were enrolled to decrease the variability in capsule anatomy from patients with pincer morphology, acetabular retroversion, or coxa profunda.
Surgical Technique
All surgeries were performed by the senior author (A.L.Z.). The periportal capsulotomy technique was used to access the hip joint.17,26 Using the anterolateral and midanterior portals, the capsule was entered and dilated to 8 mm and 10 mm, respectively. A synovectomy was performed between the portals while undermining the articular side of the capsule to increase working space without using traction sutures. The iliofemoral ligament was protected to avoid complete transection. FAIS treatment was standardized for all patients with arthroscopic labral repair performed using knotted suture anchors in a loop configuration (2 anchors) followed by femoroplasty per standard treatment for cam morphology. Patients did not undergo capsule closure.
MRI
Patients underwent preoperative and one-year postoperative magnetic resonance imaging of the affected hip using a 3 Tesla MRI scanner with an 8-channel cardiac coil (GE Healthcare, Chicago, IL). A fat suppressed, isotropic 3-dimensional intermediate-weighted fast spin echo sequence was acquired in the coronal plane, with a voxel size of 0.8 × 0.8 ×0.8 mm, field of view of 15.3 cm, echo time of 60 ms, and repetition time of 2400 to 3700 ms. The isotropic 3-dimensional sequence dataset was reconstructed using OsiriX (version 11.0.3, Pixmeo SARL) into the axial-oblique plane with a slice thickness of 3 mm and in-plane spatial resolution of 0.8 × 0.8 mm.
Image segmentation analysis
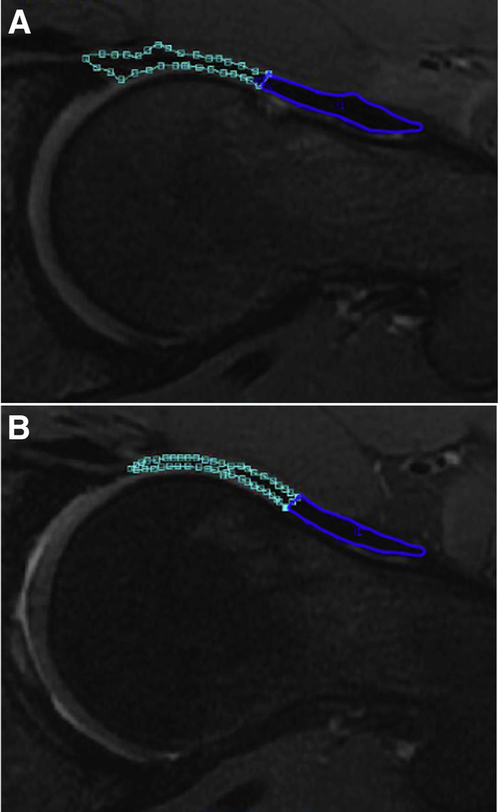
Segmentation analyses and thickness measurements were performed for each patient’s preoperative and one-year postoperative reconstructed axial-oblique MRI sequences. The 3T nonarthrographic MR was used to quantify capsule thickness because it has been shown to be highly accurate for assessment of intra-articular hip structures and to minimize effects from contrast artifact.25,27 Image segmentation was performed using the Image Processing Package (IPP) software (version 6.43.01) developed by the University of California, San Francisco Musculoskeletal Quantitative Imaging Research Group. For each sequence, three consecutive slices were identified for segmentation by selecting the slice with the widest femoral head diameter, as well as the adjacent slices directly superior and directly inferior. For each slice, a region of interest (ROI) was manually outlined, and IPP calculates the thickness corresponding to each ROI based on the number of selected voxels. The IPP software was used to calculate the ROI thickness of each slice, as well as the total thickness of each set of slices. Segmentation analysis was performed by musculoskeletal radiology trained raters on preoperative and postoperative scans to measure each patient’s anterior hip capsule thickness, posterior hip capsule thickness, anterior-posterior hip capsule thickness ratio (Fig 1), and proximal-distal anterior capsule thickness ratio which was defined at the anterior capsule midpoint (Fig 2). Intraclass correlation coefficients and 95% confidence intervals were calculated to measure intrarater reliability on the basis of a single rater, consistency, 2-way mixed effects model, and interrater reliability on the basis of a multiple rater, consistency, two-way mixed effects model. Intra-rater reliability was tested by a single rater performing segmentations on the same 10 MRI sequences 2 weeks apart and found to be 0.947 (CI, 0.785 to 0.987). Interrater reliability was tested by 2 raters on the same 10 sequences and found to be 0.922 (CI, 0.836 to 0.963).
Fig 1.
(A) Preoperative axial-oblique magnetic resonance image (MRI) of patient. (B) Preoperative MRI with anterior hip capsule outlined as the green region of interest (ROI) and posterior hip capsule outlined as the blue ROI. (C) Postoperative MRI of the same patient after femoroplasty. (D) Postoperative MRI with anterior and posterior hip capsules outlined in green and blue, respectively.
Fig 2.
(A) Preoperative and (B) postoperative magnetic resonance image of the same patient with proximal anterior hip capsule outlined as the blue region of interest (ROI) and distal anterior hip capsule outlined as the green ROI.
Patient-reported outcomes
Before surgery, patients completed the Hip Disability and Osteoarthritis Outcome Score (HOOS) including 5 subscales: pain, symptoms, activities of daily living (ADL), sport, and quality of life (QOL).28,29 One year after their surgery, patients completed the HOOS questionnaire again to assess changes in patient-reported outcomes. All data were collected on REDCap (version 7.0.19; Vanderbilt University).
Statistical Analysis
An a priori power analysis was performed with preoperative and 1-year postoperative HOOS symptoms scores on the basis of a prior study using periportal capsulotomy during hip arthroscopy.13 Twenty-four hips were needed to adequately power the study to 1 − β = 0.80 and α = 0.05. The minimal clinically important difference (MCID) for each HOOS subscale was calculated using a distribution-based method,30 where one half of the standard deviation for each subscale was used as the cutoff. The percentage of patients achieving MCID 1 year after surgery for each subscale was calculated. Preoperative and postoperative characteristics were compared using a paired sample t-test. Correlation analyses between continuous variables such as capsule thickness, capsule thickness ratios, and PRO scores were performed by calculating Pearson’s correlation coefficients and 95% confidence intervals. Statistical significance was set to P < .05. All statistical analyses were performed on IBM SPSS Statistics software.
Results
Patient cohort demographics and hip capsule characteristics
This study included 28 patients (28 hips) with a mean (±SD) age of 31.0 ± 6.1 with 16 men (57%) and 12 women (43%). Patient demographics and hip capsule morphologic characteristics at baseline and at 1-year postoperative follow-up were analyzed (Table 1). Based on Beck classification,31 intraoperative evaluation found median labral tear grade 2 (range 2–3), median acetabular cartilage grade 2 (range 2–3), and median femoral cartilage grade 1 (range 0–1). The alpha angle was significantly decreased at 1-year follow-up compared to baseline measurements (mean 44.5° vs 62.6°; P < .0001, respectively). The mean total procedure time in this cohort was 82.6 min ± 19.1 minutes. The mean traction time was 45.3 mins ± 14.1 minutes. There were no postoperative complications or reoperations in this cohort. On postoperative MRI at 1 year, all patients had findings indicating healed labral repairs without fluid signal between the labrum and acetabulum, as well as findings indicating healed periportal capsulotomies without capsular defects in the coronal or axial-oblique planes. Anterior hip capsule thickness decreased at 1-year follow-up compared to baseline thickness (mean 1395.4 mm3 vs 1758.4 mm3, P = .003, respectively). Anterior-posterior capsule thickness ratio also decreased at one-year follow-up (0.92 vs 1.12; P = 0.02, respectively). Posterior capsule thickness and proximal-distal anterior capsule thickness ratio were not significantly different at 1-year follow-up compared with baseline measurements.
Table 1.
Demographics and Characteristics (N = 28 Patients)
| Before Surgery | 1 Year After Surgery | P Value | |
|---|---|---|---|
|
| |||
| Age, y | 31.0 ± 6.1 | ||
| BMI, kg/m2 | 24.7 ± 3.1 | ||
| Male, % | 57% | ||
| Tönnis grade* | 0 | ||
| Alpha angle | 62.6° ± 5.0° | 44.5° ± 2.1° | <.001 |
| Anterior hip capsule thickness (mm3) | 1758.4 ± 487.9 | 1395.4 ± 508.4 | .003 |
| Posterior hip capsule thickness (mm3) | 1670.5 ± 435.8 | 1531.4 ± 257.9 | .08 |
| Anterior-posterior thickness ratio | 1.12 ± 0.38 | 0.92 ± 0.33 | .02 |
| Proximal-distal thickness ratio in anterior capsule | 0.68 ± 0.30 | 0.70 ± 0.27 | .65 |
Bold values indicate statistical significance.
BMI, body mass index.
All patients were Tönnis 0 in this study
Changes in PRO scores 1-year after surgery
Compared to baseline HOOS scores, all 5 HOOS subscales showed significantly increased (improved) scores at 1-year follow-up (Table 2). The HOOS QOL subscale demonstrated the greatest increase after 1 year (from 19.2 before to 61.2 after surgery, P < .001). The HOOS ADL subscale had the highest baseline (62.7 ± 18.8) and highest 1-year postoperative (86.7 ± 17.7) scores. The percentage of patients that achieved MCID for their PRO at 1-year follow-up (Table 3) ranged from 64% (HOOS symptoms) to 89% (HOOS Sports and QOL).
Table 2.
Patient-Reported Outcome Scores (N = 28 Patients)
| Before Surgery | 1 Year After Surgery | Change in Score | P Value | |
|---|---|---|---|---|
|
| ||||
| HOOS Pain | 57.7 ± 16.4 | 84.2 ± 14.8 | 26.5 ± 17.7 | <.001 |
| HOOS Symptoms | 55.7 ± 17.7 | 77.9 ± 16.0 | 22.1 ± 20.9 | <.001 |
| HOOS ADL | 62.7 ± 18.8 | 86.7 ± 17.7 | 23.9 ± 21.2 | <.001 |
| HOOS Sports | 34.4 ± 16.1 | 72.1 ± 24.8 | 37.7 ± 22.9 | <.001 |
| HOOS QOL | 19.2 ± 11.4 | 61.2 ± 24.4 | 42.0 ± 23.7 | <.001 |
Table 3.
Minimal Clinically Important Difference Thresholds
| MCID* | Percentage Achieving MCID | |
|---|---|---|
|
| ||
| HOOS Pain | 8.9 | 82% |
| HOOS Symptoms | 10.4 | 64% |
| HOOS ADL | 10.6 | 75% |
| HOOS Sports | 11.4 | 89% |
| HOOS QOL | 11.8 | 89% |
ADL, activities of daily living; HOOS, Hip Disability and Osteoarthritis Outcomes Score; MCID, minimal clinically important difference; QOL, quality of life.
MCID was calculated using the distribution-based method.
Correlating native FAIS hip capsule characteristics with baseline PRO scores
At baseline, a higher preoperative anterior to posterior hip capsule thickness ratio was correlated with lower (worse) scores on the HOOS pain (R = −0.43; P = .02; CI,−0.69 to −0.07), ADL (R = −0.43; P = .02; CI, −0.69 to −0.07), and sport (R = −0.38; P = .04; CI, −0.66 to −0.01) subscales (Fig 3). Preoperative measurements for alpha angle, anterior capsule thickness, posterior capsule thickness, and proximal to distal anterior capsule thickness ratio were not correlated with HOOS scores for any of the subscales, including pain, symptoms, ADL, sport, and QOL (Table 4).
Fig 3.
Correlating preoperative anterior-posterior hip capsule thickness ratios with Hip Disability and Osteoarthritis Outcomes Scores (HOOS). Increased preoperative anterior to posterior capsule thickness ratios were correlated with lower scores on the (A) HOOS pain (R = −0.43; P = .02; confidence interval [CI], −0.69 to −0.07), (B) HOOS ADL (R = −0.43; P = .02; CI, −0.69 to −0.07), and (C) HOOS sport (R = −0.38, P = .04, CI: −0.66 to −0.01) subscales.
Table 4.
Correlations with Preoperative Patient-Reported Outcome Scores (N = 28 Patients)
| HOOS Pain |
HOOS Symptoms |
HOOS ADL |
HOOS Sports |
HOOS QOL |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r/p | P Value | r/p | P Value | r/p | P Value | r/p | P Value | r/p | P Value | |
|
| ||||||||||
| Preoperative alpha angle | 0.08 (−0.30 to 0.44) | .70 | −0.11 (−0.47 to 0.27) | .57 | 0.04 (−0.34 to 0.41) | .84 | −0.13 (−0.48 to 0.25) | .51 | −0.19 (−0.51 to 0.22) | .35 |
| Preoperative anterior capsule thickness | −0.26 (−0.58 to 0.13) | .18 | −.02 (−0.39 to 0.36) | .92 | −0.37 (−0.65 to 0.003) | .053 | −0.25 (−0.14 to 0.25) | .20 | −0.25 (−0.57 to 0.14) | .20 |
| Preoperative posterior capsule thickness | 0.20 (−0.19 to 0.53) | .32 | 0.04 (−0.34 to 0.40) | .85 | 0.09 (−0.30 to 0.44) | .66 | 0.20 (−0.19 to 0.53) | .32 | −0.09 (−0.45 to 0.29) | .64 |
| Preoperative anterior to posterior capsule thickness ratio | −0.43 (−0.69 to −0.07) | .02 | −0.08 (−0.44 to 0.30) | .67 | −0.43 (−0.69 to −0.07) | .02 | −0.38 (−0.66 to −0.01) | .04 | −0.08 (−0.44 to 0.31) | .70 |
| Preoperative anterior capsule proximal to distal thickness ratio | −0.12 (−0.48 to 0.26) | .53 | −0.12 (−0.47 to 0.26) | .54 | −0.31 (−0.61 to 0.07) | .11 | −0.30 (−0.60 to 0.09) | .13 | −0.09 (−0.45 to 0.29) | .65 |
Bold values indicate statistical significance.
ADL, activities of daily living; HOOS, Hip Disability and Osteoarthritis Outcomes Score; QOL, quality of life.
Correlating postoperative change in hip capsule characteristics with change in PRO scores
Postoperative net change in anterior hip capsule thickness was negatively correlated with net change in HOOS ADL scores as a greater decrease from preoperative to postoperative anterior hip capsule thickness was associated with a greater increase in HOOS ADL scores (R = −0.41; P = .03; CI, −0.68 to −0.04). Postoperative net change in anterior to posterior capsule thickness ratio was negatively correlated with HOOS scores for pain (R = −0.40; P =.04; CI, −0.67 to −0.03), ADL (R = −0.45; P = .02; CI, −0.70 to −0.10), and sport (R = −0.46; P = .02; CI, −0.71 to −0.10) subscales (Fig 4). Therefore a greater decrease from preoperative to postoperative anterior to posterior capsule thickness ratio was correlated with a greater increase in HOOS pain, ADL, and sport scores. Postoperative changes in alpha angle, posterior capsule thickness, and proximal to distal anterior capsule thickness ratio were not correlated with changes in scores for any of the HOOS subscales including pain, symptoms, ADL, sport, and QOL (Table 5).
Fig 4.
Correlating postoperative change in anterior-posterior hip capsule thickness ratios with changes in HOOS Scores. A larger postoperative decrease in anterior to posterior hip capsule thickness ratio was correlated with larger postoperative increases in (A) Hip Disability and Osteoarthritis Outcomes Score (HOOS) pain (R = −0.40; P = 0.04; confidence interval [CI], −0.67 to −0.03), (B) HOOS ADL (R = −0.45; P =.02; CI, −0.70 to −0.10), and (C) HOOS sport (R = −0.46; P = 0.02; CI, −0.71 to −0.10) scores.
Table 5.
Correlations with Changes in Patient-Reported Outcome Scores (N = 28 Patients)
| Δ HOOS Pain |
Δ HOOS Symptoms |
Δ HOOS ADL |
Δ HOOS Sports |
Δ HOOS QOL |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r/p | P Value | r/p | P Value | r/p | P Value | r/p | P Value | r/p | P Value | |
|
| ||||||||||
| Δ Alpha angle | 0.28 (−0.11 to 0.59) | .16 | 0.13 (−0.26 to 0.48) | .52 | 0.34 (−0.04 to 0.63) | .08 | 0.15 (−0.24 to 0.49) | .45 | .09 (−0.30 to 0.44) | .66 |
| Δ Anterior capsule thickness | −0.33 (−0.63 to 0.05) | .08 | −0.32 (−0.62 to 0.06) | .10 | −0.41 (−0.68 to −0.04) | .03 | −0.36 (−0.65 to 0.01) | .06 | −0.37 (−0.65 to 0.01) | .05 |
| Δ Posterior capsule thickness | 0.13 (−0.26 to 0.48) | .53 | −0.006 (−0.38 to 0.37) | .97 | 0.09 (−0.30 to 0.45) | .66 | 0.29 (−0.10 to 0.60) | .14 | −0.04 (−0.40 to 0.34) | .85 |
| Δ Anterior to posterior capsule thickness ratio | −0.40 (−0.67 to −0.03) | .04 | −0.27 (−0.59 to 0.11) | .16 | −0.45 (−0.70 to −0.10) | .02 | −0.46 (−0.71 to −0.10) | .02 | −0.25 (−0.57 to 0.13) | .20 |
| Δ Anterior capsule proximal to distal thickness ratio | 0.08 (−0.30 to 0.44) | .68 | 0.23 (−0.15 to 0.56) | .23 | 0.09 (−0.29 to 0.45) | .63 | −0.01 (−0.38 to 0.36) | 0.95 | 0.13 (−0.26 to 0.48) | .52 |
Bolded values indicate statistical significance.
Δ, difference between postoperative and preoperative values; ADL, activities of daily living; HOOS, Hip Disabilitys and Osteoarthritis Outcomes Score; QOL, quality of life.
Discussion
We found that a greater preoperative anterior to posterior capsule thickness ratio was correlated with worse patient-reported scores for pain, ADL, and sport. In addition, a greater decrease from preoperative to postoperative anterior capsule thickness, relative to posterior capsule thickness, was associated with more substantial improvements in patient-reported pain, ADL, and sport scores. Last, we saw that patients treated using the periportal capsulotomy technique without capsule closure demonstrated significant improvements in PRO scores with high rates of achieving MCID and that all capsules had healed without defects by 1 year after surgery.
Findings from our study demonstrated that there were weak negative correlations between preoperative anterior to posterior hip capsule thickness ratio and HOOS pain, ADL, and sport scores (lower anterior to posterior capsule thickness ratio was correlated with higher PRO scores). This is consistent with the results of a prior study evaluating the relationship between native FAIS hip capsule morphology and PROs.25 In this study, Shaw et al.25 found that decreased anterior to posterior hip capsule volume was correlated with higher HOOS pain scores (less pain) in patients with FAIS. We found that in addition to HOOS pain, a lower preoperative anterior to posterior hip capsule thickness ratio is also correlated with higher scores on 2 other HOOS subscales: ADL and sport. However, the correlations found in our current study were weak to moderate at best and further research is needed to investigate the relationship between hip capsule morphology and patient symptoms from FAIS.
One year after arthroscopic surgery for FAIS management, the anterior hip capsule thickness, as well as the ratio of anterior to posterior hip capsule thickness, significantly decreased. These findings are likely due to the periportal capsulotomy technique, which can result in thinning of the anterior capsule during portal dilation, as well as the capsule healing with a lower anterior to posterior capsule thickness ratio compared to before surgery. The postoperative net change in anterior to posterior hip capsule thickness ratio also demonstrated weak but statistically significant negative correlations with 3 HOOS subscale scores: pain, ADL, and sport (greater decrease in anterior to posterior capsule ratio was correlated with greater PRO score improvements). One prior anatomical study suggested that a thicker iliofemoral ligament in the anterior hip capsule was associated with the development of cam morphology32 and decreased range of motion during hip flexion and internal rotation, which if valid, could explain some of the findings in our current study.33 In addition, a thicker anterior capsule could theoretically exacerbate the posterior hip instability/microinstability that has been associated with FAIS,34,35 especially in patients with a thinner posterior capsule. This study cannot draw conclusions on these theories but only serve to elicit further studies to assess the mechanisms under which capsule morphology may contribute to patient outcomes after surgery.
There lacks a consensus on whether capsular closure is necessary after hip arthroscopy for FAIS management; some studies advocate for tight capsule repair20,21 whereas others argue that it is not needed.16,19,36 For our study, all 28 hips that underwent periportal capsulotomy without capsule closure showed continuous capsular healing without any signs of defect on MRI 1 year after surgery. Strickland et al.37 showed that the hip capsule was healed by 24 weeks after surgery with interportal capsulotomy. Moreover, Chambers et al.17 showed significant clinical improvement after 2 years using periportal capsulotomy without capsule closure. Our findings further support that capsular closure may not be necessary in patients without joint hypermobility using a periportal capsulotomy approach and that debulking anterior capsule thickness may contribute to improvements in patient outcomes.
Last, the alpha angle is used as a quantitative measure of cam morphology and a higher alpha angle has been associated with increased labral tear grade and acetabular cartilage damage.38,39 Although one previous study found a negative correlation between preoperative and postoperative alpha angle with PRO scores,40 other studies did not find this association between lower postoperative alpha angles and improved clinical outcomes.41,42 Our results did not reveal a significant correlation between postoperative decrease in alpha angle and change in HOOS scores. This is likely due to uniform femoroplasty corrections because all surgeries were performed by a single surgeon using the same technique in this cohort. All patients had uniformly high preoperative alpha angles when enrolled and after femoroplasty, all patients had similar postoperative alpha angles. Therefore, because there was little variability in the amount of change from preoperative and postoperative alpha angles, an association was not found in this study between changes in alpha angle and changes in HOOS scores.
Limitations
This study is subject to several limitations. First, our segmentation technique relies on thickness calculations of 2-dimensional MRI slices to study the morphology of the hip capsule, a 3-dimensional structure. Postoperative MRIs were performed without arthrography, which is a limitation in evaluating complete capsule healing. In addition, our study did not include a cohort of patients that underwent capsular repair. Therefore we could not assess how capsular repair affects postoperative hip capsule morphology and symptoms for patients who underwent capsulotomy. This study also only evaluated cam morphology FAIS to decrease the variability in capsule anatomy, but we aim to have future studies assess hip capsule characteristics for pincer morphology FAIS, as well as the effects of capsule repair. All arthroscopic surgeries were performed at a single hospital by a single surgeon, which may limit generalizability of the findings, but a standardized surgical procedure may help to ensure reproducibility of the results. Last, this study reports 1-year outcomes after surgery. Prolonged follow-up duration to 2 or more years may demonstrate further changes in results, and this is a goal for future studies. Our 1-year assessment of capsular healing was still important because it showed that within this time, capsulotomies were able to heal without signs of defects, and this may be valuable data to aid in clinical counseling for patients.
Conclusion
Periportal capsulotomy without closure demonstrates capsule healing by 1 year after arthroscopic FAIS treatment. Changes in hip capsule morphology including decreased anterior-posterior capsule thickness ratio after surgery may be correlated with improvements in patient pain, function, and ability to return to sports.
Supplementary Material
Acknowledgments
The authors report the following potential conflicts of interest or sources of funding: Supported by NIH/NIAMS grant number P50 AR060752, and the American Orthopaedic Society for Sports Medicine (YIG-2016-1). A.L.Z. reports grants from the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases and the American Orthopaedic Society for Sports Medicine; personal fees from Stryker; board or committee membership for the American Orthopaedic Society for Sports Medicine; research support from Zimmer; education support from Arthrex. T.P.V. reports personal fees from Depuy Synthes and Medical Device. T.M.L reports personal fees from Pfizer and Regeneron; nonfinancial support from GE Healthcare and Medtronic. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Contributor Information
Kevin H. Nguyen, Department of Orthopaedic Surgery, University of California–San Francisco, San Francisco
Chace Shaw, Department of Orthopaedic Surgery, University of California–San Francisco, San Francisco
Thomas M. Link, Musculoskeletal and Quantitative Imaging Research Group, Department of Radiology and Biomedical Imaging, University of California–San Francisco, San Francisco, California, U.S.A.
Sharmila Majumdar, Musculoskeletal and Quantitative Imaging Research Group, Department of Radiology and Biomedical Imaging, University of California–San Francisco, San Francisco, California, U.S.A.
Richard B. Souza, Musculoskeletal and Quantitative Imaging Research Group, Department of Radiology and Biomedical Imaging, University of California–San Francisco, San Francisco, California, U.S.A.
Thomas P. Vail, Department of Orthopaedic Surgery, University of California–San Francisco, San Francisco
Alan L. Zhang, Department of Orthopaedic Surgery, University of California–San Francisco, San Francisco
References
- 1.Ganz R, Parvizi J, Beck M, Leunig M, Nötzli H, Siebenrock KA. Femoroacetabular iImpingement: A cause for osteoarthritis of the hip. Clin Orthop Relat Res 2003;417: 112–120. [DOI] [PubMed] [Google Scholar]
- 2.Sankar WN, Nevitt M, Parvizi J, Felson DT, Agricola R, Leunig M. Femoroacetabular impingement: Defining the condition and its role in the pathophysiology of osteoarthritis. J Am Acad Orthop Surg 2013;21:S7–S15. [DOI] [PubMed] [Google Scholar]
- 3.Kuhns BD, Weber AE, Levy DM, Wuerz TH. The natural history of femoroacetabular impingement. Front Surg 2015;2:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wyles CC, Norambuena GA, Howe BM, et al. Cam deformities and limited hip range of motion are associated with early osteoarthritic changes in adolescent athletes: A prospective matched cohort study. Am J Sports Med 2017;45:3036–3043. [DOI] [PubMed] [Google Scholar]
- 5.Agricola R, Heijboer MP, Bierma-Zeinstra SMA, Verhaar JAN, Weinans H, Waarsing JH. Cam impingement causes osteoarthritis of the hip: a nationwide prospective cohort study (CHECK). Ann Rheum Dis 2013;72: 918–923. [DOI] [PubMed] [Google Scholar]
- 6.Ng VY, Arora N, Best TM, Pan X, Ellis TJ. Efficacy of surgery for femoroacetabular impingement: A systematic review. Am J Sports Med 2010;38:2337–2345. [DOI] [PubMed] [Google Scholar]
- 7.Rhon DI, Greenlee TA, Sissel CD, Reiman MP. The two-year incidence of hip osteoarthritis after arthroscopic hip surgery for femoroacetabular impingement syndrome. BMC Musculoskelet Disord 2019;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bozic KJ, Chan V, Valone FH, Feeley BT, Vail TP. Trends in hip arthroscopy utilization in the United States. J Arthroplasty 2013;28:140–143. [DOI] [PubMed] [Google Scholar]
- 9.Clohisy JC, St John LC, Schutz AL. Surgical treatment of femoroacetabular impingement: A systematic review of the literature. Clin Orthop Relat Res 2010;468:555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Degen RM, Mayer SW, Fields KG, Coleman SH, Kelly BT, Nawabi DH. Functional outcomes and cam recurrence after arthroscopic treatment of femoroacetabular impingement in adolescents. Arthroscopy 2017;33: 1361–1369. [DOI] [PubMed] [Google Scholar]
- 11.Gohal C, Shamshoon S, Memon M, et al. Health-related quality of life after hip arthroscopy for femoroacetabular impingement: A systematic review and meta-analysis. Sports Health 2019;11:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sing DC, Feeley BT, Tay B, Vail TP, Zhang AL. Age-related trends in hip arthroscopy: A large cross-sectional analysis. Arthroscopy 2015;31:2307–2313. [DOI] [PubMed] [Google Scholar]
- 13.Flores SE, Sheridan JR, Borak KR, Zhang AL. When do patients improve after hip arthroscopy for femoroacetabular impingement? A prospective cohort analysis. Am J Sports Med 2018;46:3111–3118. [DOI] [PubMed] [Google Scholar]
- 14.Maldonado DR, Yelton MJ, Rosinsky PJ, et al. Return to play after hip arthroscopy among tennis players: outcomes with minimum five-year follow-up. BMC Musculoskelet Disord 2020:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mygind-Klavsen B, Kraemer O, Hölmich P, Lund B. An updated description of more than 5,000 procedures from the Danish Hip Arthroscopy Registry. J Bone Joint Surg Am 2020;102:43–50. [DOI] [PubMed] [Google Scholar]
- 16.Ekhtiari S, de SA D, Haldane CE, et al. Hip arthroscopic capsulotomy techniques and capsular management strategies: A systematic review. Knee Surg Sports Traumatol Arthrosc 2017;25:9–23. [DOI] [PubMed] [Google Scholar]
- 17.Chambers CC, Monroe EJ, Flores SE, Borak KR, Zhang AL. Periportal capsulotomy: Technique and outcomes for a limited capsulotomy during hip arthroscopy. Arthroscopy 2019;35:1120–1127. [DOI] [PubMed] [Google Scholar]
- 18.Camp CL, Reardon PJ, Levy BA, Krych AJ. Creating and closing the T-capsulotomy for improved visualization during arthroscopic treatment of femoroacetabular impingement. Arthrosc Tech 2015;4:731–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filan D, Carton P. Routine interportal capsular repair does not lead to superior clinical outcome following arthroscopic femoroacetabular impingement correction with labral repair. Arthroscopy 2020;36:1323–1334. [DOI] [PubMed] [Google Scholar]
- 20.Frank RM, Lee S, Bush-Joseph CA, Kelly BT, Salata MJ, Nho SJ. Improved outcomes after hip arthroscopic surgery in patients undergoing T-capsulotomy with complete repair versus partial repair for femoroacetabular impingement: A comparative matched-pair analysis. Am J Sports Med 2014;42:2634–2642. [DOI] [PubMed] [Google Scholar]
- 21.Hassebrock JD, Makovicka JL, Chhabra A, et al. Hip arthroscopy in the high-level athlete: Does capsular closure make a difference? Am J Sports Med 2020;48: 2465–2470. [DOI] [PubMed] [Google Scholar]
- 22.Rakhra KS, Bonura AA, Nairn R, Schweitzer ME, Kolanko NM, Beaule PE. Is the hip capsule thicker in diseased hips? Bone Joint Res 2016;5:586–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kay J, Memon M, Rubin S, et al. The dimensions of the hip capsule can be measured using magnetic resonance imaging and may have a role in arthroscopic planning. Knee Surg Sports Traumatol Arthrosc 2020;28:1246–1261. [DOI] [PubMed] [Google Scholar]
- 24.Magerkurth O, Jacobson JA, Morag Y, Caoili E, Fessell D, Sekiya JK. Capsular laxity of the hip: Findings at magnetic resonance arthrography. Arthroscopy 2013;29:1615–1622. [DOI] [PubMed] [Google Scholar]
- 25.Shaw C, Warwick H, Nguyen KH, et al. Correlation of hip capsule morphology with patient symptoms from femoroacetabular impingement. J Orthop Res 2021;39:590–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monroe EJ, Chambers CC, Zhang AL. Periportal capsulotomy: A technique for limited violation of the hip capsule during arthroscopy for femoroacetabular impingement. Arthrosc Tech 2019;8:e205–e208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linda DD, Naraghi A, Murnaghan L, Whelan D, White LM. Accuracy of non-arthrographic 3T MR imaging in evaluation of intra-articular pathology of the hip in femoroacetabular impingement. Skeletal Radiol 2017;46: 299–308. [DOI] [PubMed] [Google Scholar]
- 28.Kemp JL, Collins NJ, Roos EM, Crossley KM. Psychometric properties of patient-reported outcome measures for hip arthroscopic surgery. Am J Sports Med 2013;41: 2065–2073. [DOI] [PubMed] [Google Scholar]
- 29.Nilsdotter A, Bremander A. Measures of hip function and symptoms: Harris Hip Score (HHS), Hip Disability and Osteoarthritis Outcome Score (HOOS), Oxford Hip Score (OHS), Lequesne Index of Severity for Osteoarthritis of the Hip (LISOH), and American Academy of Orthopedic Surgeons (AAOS) Hip and Knee Questionnaire. Arthritis Care Res 2011;63:S200–S207. [DOI] [PubMed] [Google Scholar]
- 30.Harris JD, Brand JC, Cote MP, Faucett SC, Dhawan A. Research Pearls: The significance of statistics and perils of pooling. Part 1: Clinical versus statistical significance. Arthroscopy 2017;33:1102–1112. [DOI] [PubMed] [Google Scholar]
- 31.Beck M, Kalhor M, Leunig M, Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br 2005;87: 1012–1018. [DOI] [PubMed] [Google Scholar]
- 32.Lee CB, Spencer HT, Nygaard KF. Femoral cam deformity due to anterior capsular force: A theoretical model with MRI and cadaveric correlation. J Orthop 2016;13:331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang K, de Sa D, Yu H, Choudur HN, Simunovic N, Ayeni OR. Hip capsular thickness correlates with range of motion limitations in femoroacetabular impingement. Knee Surg Sports Traumatol Arthrosc 2018;26:3178–3187. [DOI] [PubMed] [Google Scholar]
- 34.Canham CD, Yen Y-M, Giordano BD. Does femoroacetabular impingement cause hip instability? A systematic review. Arthroscopy 2016;32:203–208. [DOI] [PubMed] [Google Scholar]
- 35.Krych AJ, Thompson M, Larson CM, Byrd JWT, Kelly BT. Is posterior hip instability associated with cam and pincer deformity? Clin Orthop Relat Res 2012;470:3390–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atzmon R, Sharfman ZT, Haviv B, et al. Does capsular closure influence patient-reported outcomes in hip arthroscopy for femoroacetabular impingement and labral tear? J Hip Preserv Surg 2019;6:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strickland CD, Kraeutler MJ, Brick MJ, et al. MRI evaluation of repaired versus unrepaired interportal capsulotomy in simultaneous bilateral hip arthroscopy: A double-blind, randomized controlled trial. J Bone Joint Surg Am 2018;100:91–98. [DOI] [PubMed] [Google Scholar]
- 38.Grace T, Samaan MA, Souza RB, Link TM, Majumdar S, Zhang AL. Correlation of patient symptoms with labral and articular cartilage damage in femoroacetabular impingement. Orthop J Sports Med 2018;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laborie LB, Lehmann TG, Engesæter IØ, Sera F, Engesæter LB, Rosendahl K. The alpha angle in cam-type femoroacetabular impingement. Bone Joint J 2014;96: 449–454. [DOI] [PubMed] [Google Scholar]
- 40.Lansdown DA, Kunze K, Ukwuani G, Waterman BR, Neal WH, Nho SJ. Pre-operative and post-operative alpha angles are significant independent predictors of patient-reported outcome measures at two years after hip arthroscopy. Orthop J Sports Med 2018;6. [Google Scholar]
- 41.Briggs KK, Soares E, Bhatia S, Philippon MJ. Postoperative alpha angle not associated with patient-centered midterm outcomes following hip arthroscopy for FAI. Knee Surg Sports Traumatol Arthrosc 2019;27: 3105–3109. [DOI] [PubMed] [Google Scholar]
- 42.Fairley J, Wang Y, Teichtahl AJ, et al. Management options for femoroacetabular impingement: A systematic review of symptom and structural outcomes. Osteoarthritis Cartilage 2016;24:1682–1696. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.