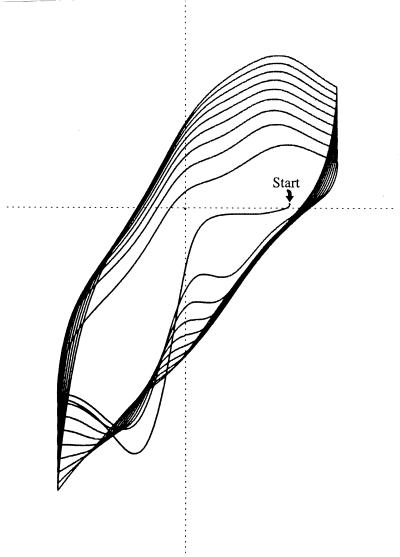
FIG. 4.
Redox buffering by melanin. The electrochemical potential of an inert electrode was rhythmically cycled between positive and negative (indicated on horizontal axis) in the presence of the dissolved melanin precursor, 5,6-dihydroxyindole. The precursor was incrementally oxidized and melanin was incrementally precipitated with each positive sweep. Current to and from the electrode was recorded on the vertical axis. Because voltage varied linearly with time, the horizontal “voltage axis” is also a time axis. Since charge equals current times time and is proportional to area, the increasing area circumscribed indicates that buffer capacity increases with melanin film thickness (22). Reprinted from reference 29.