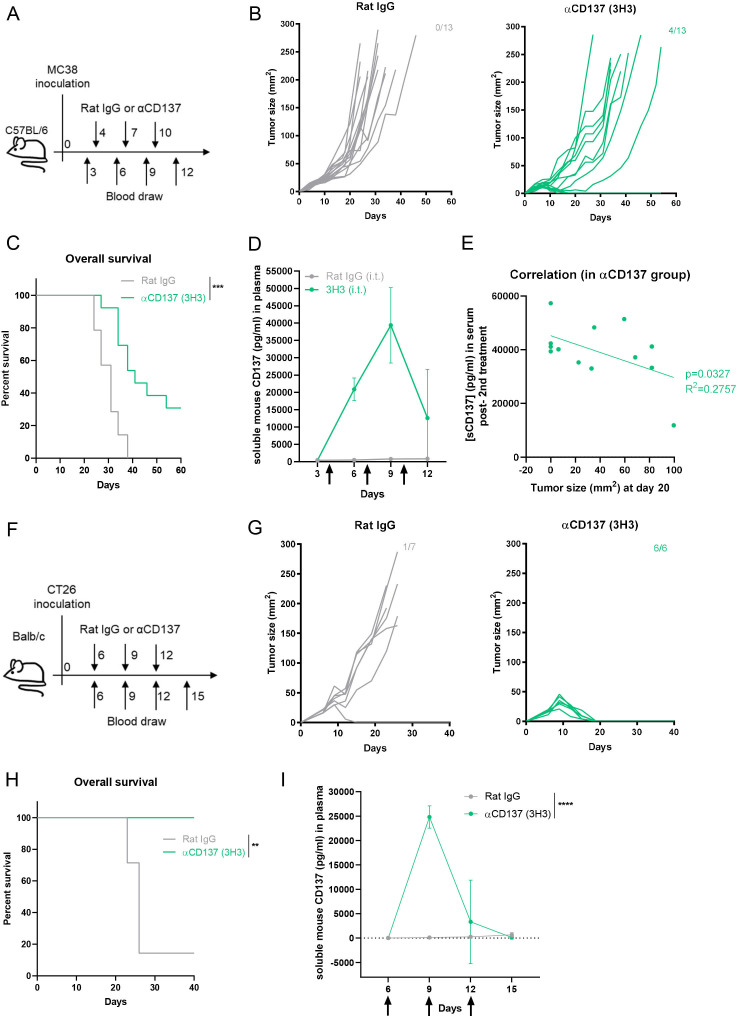
Figure 3.
sCD137 increases in peripheral blood of tumor-bearing mice undergoing treatment with agonist anti-CD137 mAb. (A) Experimental treatment and blood sample collection in mice bearing MC38-derived tumors. (B) Tumor size follow-up and fractions of complete rejections attained in mice (n=13 per group) treated with anti-CD137 mAb or control antibody and the corresponding overall survival (C) are shown. (D) Plasma sCD137 concentrations (mean±SD) in sequential follow-up overtime measured by ELISA in the groups of mice treated as indicated. (E) Plotted statistical correlation of plasma sCD137 on day +9 since tumor cell inoculation and the size of the MC38-derived tumors on day +20. (F) Similar experimental design in Balb/c mice bearing syngeneic CT26 tumors. (G) Individual tumor size follow-up and fractions of mice that completely rejected their tumors. (H) Overall survival of such mice. (I) Plasma sCD137 concentrations (mean±SD) in sequential follow-up overtime of the mice treated in part F. Statistical significance was assessed by log-rank (Mantel-Cox) test in figure parts C and H, and two-way ANOVA in figure parts D and I. *p<0.05, **p<0.01, ****p<0.0001. ANOVA, analysis of variance; ns, not significant.