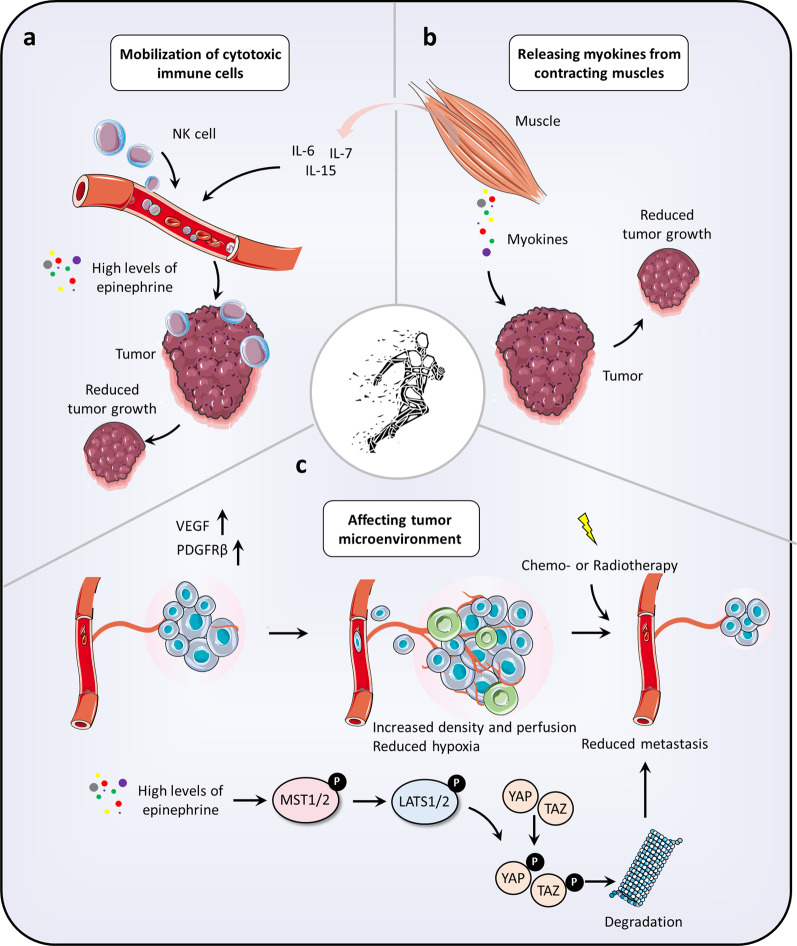
Fig. 4.
The interplay between activity–rest cycles and cancer. a activity–rest cycles regulate immune function for tumor suppression. Exercise has been found to promote immune cell infiltration by epinephrine-driven NK cells circulation, resulting in a marked inhibition of cancer. Moreover, exercise also enhances naïve T cell populations and mitigates detrimental effects on T cells, leading to more efficient generation of immunological memory. b activity–rest cycles regulate the crosstalk between muscle and tumor. The crosstalk between muscle and tumor depends on skeletal muscle-secreted myokines. Exercise-induced myokines, including OSM, irisin, and SPARC, may play a role in cancer prevention and therapy. In addition, exercise-induced myokines can also induce the secretion of immune regulatory cytokines, including IL-6, IL-7, and IL-15, which indirectly regulate immune cell function. c, activity–rest cycles regulate tumor microenvironment. Exercise-mediated promotion of pro-angiogenic cytokines (such as VEGF) induces vascular remodeling to increase density and perfusion and reduce hypoxia in the tumor microenvironment. Furthermore, exercise was involved in the regulation of the Hippo signaling pathway, in which exercise-driven epinephrine markedly inhibited tumor formation by inducing Yap and Taz phosphorylation and subsequent degradation. NK cells: natural killer cells; OSM: oncostatin M; SPARC: secreted protein acidic and rich in cysteine; VEGF: vascular endothelial growth factor