Abstract
Purpose
Panobinostat, an orally bioavailable pan-HDAC inhibitor, has demonstrated potent activity in multiple malignancies, including pediatric brain tumors such as DIPG, with increased activity against H3K27M mutant cell lines. Given limited evidence regarding the CNS penetration of panobinostat, we sought to characterize its BBB penetration in a murine model.
Methods
Panobinostat 15 mg/kg was administered IV to 12 CD-1 female mice. At specified time points, mice were euthanized, blood samples were collected, and brains were removed. LC–MS was performed to quantify panobinostat concentrations. Cmax and AUC were estimated and correlated with previously published pharmacokinetic analyses and reports of IC-50 values in DIPG cell lines.
Results
Mean panobinostat plasma concentrations (ng/mL) were 27.3 ± 2.5 at 1 h, 7.56 ± 1.8 at 2 h, 1.48 ± 0.56 at 4 h, and 2.33 ± 1.18 at 7 h. Mean panobinostat brain concentrations (ng/g) were 60.5 ± 6.1 at 1 h, 42.9 ± 5.4 at 2 h, 33.2 ± 6.1 at 4 h, and 28.1 ± 4.3 at 7 h. Brain-to-plasma ratio at 1 h was 2.22 and the brain to plasma AUC ratio was 2.63. Based on the published human pharmacokinetic data, the anticipated Cmax in humans is expected to be significantly higher than the IC-50 identified in DIPG models.
Conclusion
It is expected that panobinostat would be effective in CNS tumors where the IC-50 is in the low nanomolar range. Thus, our data demonstrate panobinostat crosses the BBB and achieves concentrations above the IC-50 for DIPG and other brain tumors and should be explored further for clinical efficacy.
Keywords: Panobinostat, Pharmacokinetics, Brain penetration, In vivo, DIPG
Introduction
Epigenetic alterations via histone deacetylase (HDAC) activity play an important role in oncogenesis, and this enzyme represents a prime target for cancer treatment [1]. HDACs modulate the acetylation of histones in nucleosomes and non-histone substrates, such as tumor suppressor genes and oncogenes [1–4]. Acetylation of histone and non-histone proteins alters chromatin configuration, resulting in regulation of gene expression. Mutations of HDAC genes, resulting in altered expression and downregulation of HDAC, have been associated with tumor development. HDAC inhibitors regulate acetylation by targeting HDAC, leading to transcription of tumor suppressor genes [2–5].
Several HDAC inhibitors are clinically available or in development, with varying affinity for the HDAC isozymes [5]. Panobinostat is an orally bioavailable pan-HDAC inhibitor which has demonstrated potent activity in multiple malignancies and is currently FDA approved for relapsed and refractory multiple myeloma [6, 7]. Of particular interest is the high potency of panobinostat compared to other HDAC inhibitors in pediatric brain tumor cell lines [3, 8]. Brain and other nervous system tumors account for 26% of malignancies in children, second only to leukemia [9]. For individuals less than 20 years of age, brain and other nervous system tumors are the leading cause of cancer-related death. For many pediatric brain tumors, especially high-grade gliomas, traditional chemotherapy has not significantly improved clinical outcomes [10]. Thus, additional targeted therapy options, including those targeting epigenetic pathways, are urgently needed.
Fortunately, pediatric high-grade gliomas are more likely to harbor genetic mutations than their adult counterparts, offering targeted therapy as a potential therapeutic option [10]. Histone H3 mutations occur in 36% of pediatric glioblastomas. Approximately 59.7% of diffuse midline gliomas (DMGs) and 63% of diffuse intrinsic pontine gliomas (DIPGs), a subset of DMGs, harbor H3K27M mutations [11]. Panobinostat has demonstrated efficacy against brainstem glioma cells both in vitro and in vivo, with increased activity against H3K27M-mutant cell lines [3, 12]. In a study evaluating the activity of 83 drugs in DIPG cell lines, panobinostat was one of the most effective agents, with 12 of the 16 DIPG cultures demonstrating sensitivity [12].
Despite promising pre-clinical activity of panobinostat in pediatric brain tumors and other malignancies with a proclivity for CNS involvement, there is limited evidence to suggest panobinostat crosses the blood–brain barrier (BBB). Physiochemical parameters associated with BBB penetration include a molecular weight less than 500 g/mol, adequate lipophilicity (logP), low protein binding, and lack of affinity for efflux pumps, such as p-glycoprotein (P-gp) and breast cancer resistance protein (BCRP) [13, 14]. Panobinostat has a molecular weight of 349.4 g/mol and is lipophilic (consensus log Po/w = 2.98),but it is 90% protein bound and is a substrate of P-gp. In addition, clinical reports have suggested that panobinostat exhibits minimal to no brain penetration [15–18]. Given the variable evidence, we sought to evaluate the concentration of panobinostat in the plasma and brain in a murine model.
Materials and methods
Chemicals and reagents
Panobinostat [2-hydroxypropanoic acid, compound with 2-(E)-Nhydroxy-3-[4-[[[2-(2-methyl-1H-indol-3-yl)ethyl] amino]methyl] phenyl]-2-propenamide] was purchased from Cayman Chemical Company (Ann Arbor, MI, USA). Panobinostat was compounded into a 3 mg/mL solution using a phosphate-buffered saline (PBS) containing 20% dimethyl sulfoxide (DMSO) and 20% PEG-400. Stock solutions of panobinostat were prepared in methanol and were serially diluted with the respective biomatrix (plasma and brain homogenate) to generate the calibration standards (1–2000 ng). Blank plasma from a different source was used to dilute quality control (QC) working solutions to obtain QC plasma and brain homogenate samples at 5, 100, and 500 ng/mL. Dilutions of the internal standard (IS), CE302 (stock in acetonitrile) were prepared at 400 ng/mL in the respective biomatrix.
Mice and drug administration
Pharmacokinetic evaluation of panobinostat was performed using twelve CD-1 female mice that were 8–10 weeks of age. All mice were handled according to the guidelines approved by the University Committee on Use and Care of Animals (UCUCA) and housed in the Unit for Laboratory Animal Medicine (ULAM), an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) accredited facility. All supervisors are certified by the American Association for Laboratory Animal Science (AALAS). Veterinary care was provided by ULAM faculty and veterinary residents in accordance with the Guide for the Care and Use of Laboratory Animals [19]. Panobinostat at doses of 15 mg/kg was administered by intravenous (IV) injection (Fig. 1). Doses ranging from 10 mg/kg to 20 mg/kg have been used in prior xenograft studies [3].
Fig. 1.
Pharmacokinetic experimental design. The figure provides an overview of drug administration and subsequent experimental steps of this study. Twelve mice were administered 15 mg/kg panobinostat intravenously at time-point 0. Three mice from each treatment group were killed at each time-point of 1, 2, 4, and 7 h after injection. Once killed, brain tissue and plasma samples were obtained, processed and analyzed by liquid chromatography–mass spectroscopy to determine drug concentrations, and then analyzed for pharmacokinetic parameters. Figure created with BioRender.com. Abbreviations: TVI tail vein injection, LC–MS liquid chromatography–mass spectroscopy, PK pharmacokinetic
Plasma and brain concentration–time profile
The mice were euthanized using CO2 or exsanguination under anesthesia at four pre-specified time points (1 h, 2 h, 4 h, and 7 h). Three mice were killed at each time-point (Fig. 1). Blood samples were collected using heparinized 1 mL syringes with 25 g*1 needles. Samples were then centrifuged at 15,000 rpm for 10 min. Plasma was collected from the upper layer and frozen at − 80 °C immediately after blood collection. Brains were removed at the specified time points and frozen at − 80 °C for later analysis. Brain homogenate samples were prepared by homogenizing the tissue, 4 times for 20 s each time at 6500 RPM in a Precellys Evolution system (Montigny-le-Bretonneux, France).
Assay methodology
Liquid chromatography–mass spectrometry (LC–MS) method development was performed using an API 5500TM Q-trap tandem mass spectrometer (Applied Biosystems) operating with Turbo-Ionspray™ Interface and positive or negative ion-mode multiple-reaction monitoring (MRM). The MRM transitions were predicted a priori based on MS conditions. Chromatography with blank plasma and blank plasma spiked with IS was performed to demonstrate that blank plasma had no interference to panobinostat or IS determination. This process was repeated with blank brain homogenate and blank brain homogenate spiked with IS. Mobile Phase A and Mobile Phase B consisted of 0.1% formic acid in purified deionized water and 0.1% formic acid in acetonitrile, respectively. Calibration curves were used to quantify panobinostat in mouse plasma and brain. The curves for both plasma and brain samples were constructed using 9 nonzero standards ranging from 1 to 500 ng/mL, after excluding for contamination or interference with a blank sample. The curves were built with linear regression (1/x2).
Pharmacokinetic and statistical analyses
The maximum concentration (Cmax) was the highest concentration observed, which was at the first time-point of 1 h post dose administration (C1). The trapezoidal rule was used to compute the area under the curve from first (1 h) to last (7 h) measurable sample (AUC1–7). The nonparametric adaptive grid algorithm was used by evoking the PMetrics® library through R to perform compartmental pharmacokinetic analysis. Individual animals contributed a single blood and brain sample per time-point that were pooled together for analysis. The initial model structure included a two-compartment system with bolus intravenous input, first-order transfer rate constants, and clearance. Models of higher complexity were tested and discrimination between models determined by goodness-of-fit plots and the Akaikie Information Criterion. The results were compared to literature and pre-clinical information contained within regulatory documents accessed through the drugs@fda.gov portal. These details were used to correlate the results of this study with IC-50 values that have been previously reported and to compare to pharmacokinetic analyses in humans.
Results
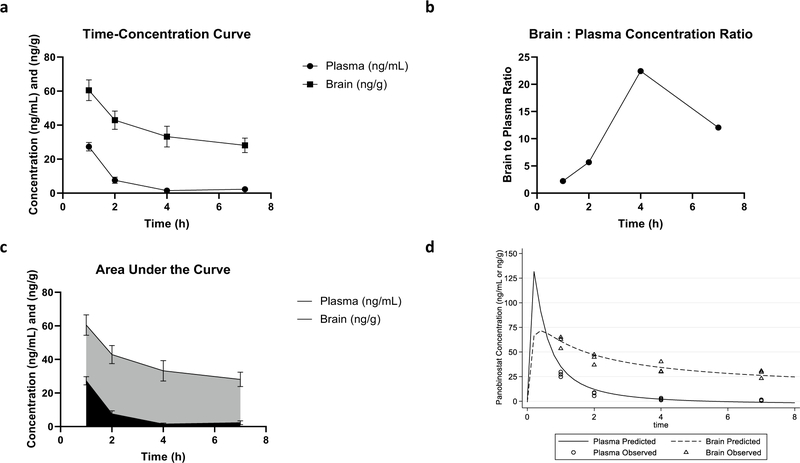
The median weight of the 12 mice was 28.4 g, and median brain weight was 0.431 g. All mice received a dose of panobinostat IV at 15 mg/kg. The LC–MS method was established with strong correlation coefficients for plasma (r = 0.9992) and brain (r = 0.9997) between peak area ratio and concentration for quantification. The accuracy of QC samples were within 15% of the nominal concentrations and the coefficient of variation ≤ 15% (precision). Mean panobinostat plasma concentrations (ng/mL) were 27.3 ± 2.5 at 1 h, 7.56 ± 1.8 at 2 h, 1.48 ± 0.56 at 4 h, and 2.33 ± 1.18 at 7 h (Fig. 2a). Mean panobinostat brain concentrations (ng/g) were 60.5 ± 6.1 at 1 h, 42.9 ± 5.4 at 2 h, 33.2 ± 6.1 at 4 h, and 28.1 ± 4.3 at 7 h (Fig. 2a). The brain to plasma concentration ratio at each time period is displayed in Fig. 2b. The ratio of brain to plasma C1 was 2.22. The panobinostat AUC1–7 for plasma was 95.0 h.ng/mL and 250 h.ng/g for brain (Fig. 2c). The ratio of brain to plasma AUC1–7 was 2.63. A three-compartment model provided the best fit to the data with a median clearance, volume of distribution of the plasma compartment, and volume of distribution of the brain compartment of 81.7 L/kg•h, 139.7 L/kg, and 49.8 L/kg, respectively. The model structure, observed and predicted plots, convergence and support point, and final parameter estimates are included in the supplemental file, which demonstrate an excellent model fit to the data for plasma and brain. Figure 2d illustrates the model predicted (based on simulations from the final model) and observed concentrations in plasma and brain. The estimated Cmax in plasma and brain in this animal model with 15 mg/kg is 131.7 ng/mL and 72.4 ng/g, respectively.
Fig. 2.
Pharmacokinetic profile of panobinostat. Pharmacokinetic profile of panobinostat in brain and plasma. a Time–concentration curve of panobinostat concentrations in brain and plasma. Mean concentrations (ng/mL) of panobinostat in the plasma (circle) were 27.3 ± 2.5 at 1 h, 7.56 ± 1.8 at 2 h, 1.48 ± 0.56 at 4 h, and 2.33 ± 1.18 at 7h. Mean concentrations (ng/g) of panobinostat in the brain (square) were 60.5 ± 6.1 at 1 h, 42.9 ± 5.4 at 2 h, 33.2 ± 6.1 at 4 h, and 28.1 ± 4.3 at 7 h. b Brain:Plasma concentration ratios of panobinostat over time. Ratios of brain panobinostat concentration to plasma panobinostat concentration were 2.22 at 1 h, 5.67 at 2 h, 22.43 at 4 h, and 12.06 at 7 h. c Plasma and brain area under the curve (AUC) profile for panobinostat in plasma and brain. The AUC from hour 1 to hour 7 of plasma (black) was 95.0 h.ng/mL and brain (gray) was 250 h.ng/g. d Observed and model predicted (median) plasma and brain concentrations of panobinostat in mice. The estimated Cmax in plasma and brain with a 15 mg/kg IV dose is 131.7 ng/mL and 72.4 ng/g, respectively. Figures created with GraphPad Prism. Abbreviations: Cmax = maximum concentration, IV intravenous
A pharmacokinetic analysis of panobinostat in pediatric patients identified a median Cmax of 20.7 ng/mL following oral doses of 20 mg/m2 [20]. Assuming the brain to plasma ratio of 2.22 identified in our study, it is expected that human brain concentrations would be approximately 45.95 ng/g. The corresponding nanomolar concentration would be approximately 142.2 nM [21]. This value is 5 times above the IC-50 of 28.3 nM identified in prior DIPG models [3]. An adult pharmacokinetic study has identified similar Cmax values, ranging from 10.8 ng/mL to 21.6 ng/mL, following oral doses of panobinostat 20 mg, the recommended dose for adults with multiple myeloma [22, 23]. If administered at these identified doses, it is expected that panobinostat would be effective in CNS tumors where the IC-50 is below 142.2 nM.
Discussion
Our data demonstrate adequate panobinostat concentrations in brain tissue in mice after a 15 mg/kg dose. The mean peak concentration one hour after administration was 60.5 ng/g, and a detectable concentration remained at seven hours after administration with a mean concentration of 28.1 ng/g. The mouse brain has been reported to be slightly denser than water at 1.0448 ± 0.0016 g/mL [24]. If we assume this applies to the mice used in our study, the 1 and 7 h concentrations would correspond to 63.2 ng/mL and 29.4 ng/mL, respectively, and further to nanomolar concentrations of 180.9 nM and 84.1 nM, respectively. Prior studies have reported IC-50 s ranging from 12.1–28.3 nM for panobinostat in human DIPG cell lines [3]. Therefore, the concentrations identified in our study would be expected to exceed the reported IC-50 s. There are certainly limitations to the extrapolation of this data. For example, the aforementioned density applies to the cerebral cortex of mouse brain, while the IC-50 s refer to DIPG cell lines, which originate in the brainstem.
These results contrast what was expected based on physiochemical properties and what has been reported in other pre-clinical studies [15–18]. Panobinostat is highly protein bound and is a substrate of P-gp, which was thought to limit its BBB penetration. However, a web-based pharmacokinetic tool, the BIOLED (Brain Or IntestinaL EstimateD permeation)-Egg model (http://www.swissadme.ch/index.php), which predicts BBB penetration by computing the lipophilicity and polarity of small molecules, also predicted brain penetration of panobinostat [25]. Our murine pharmacokinetic results are consistent with the BOILED-Egg model and suggest panobinostat crosses the BBB due to its high lipophilicity (consensus Log P = 2.98), low number of H-bond donors and acceptors, and relatively low total polar surface area (77.15 Å2) despite its affinity for P-gp. Inhibition of P-gp efflux could potentially increase concentrations of panobinostat in the brain even further. This concept has been shown to be successful with increased concentrations of dasatinib and vandetanib in the brain after inhibition of P-gp with the m-TOR inhibitor, everolimus [26, 27].
Prior studies have suggested agents that the permeability and solubility characteristics of drugs (classified according to the Biopharmaceutical Classification System) may determine the extent to which efflux pumps limit gastrointestinal membrane absorption [28–30]. For example, drugs with high permeability and high solubility (BCS Class I drugs) are less impacted by absorption-related drug interactions due to p-glycoprotein efflux, as the permeability of the compound exceeds the impact of active efflux, possibly due to saturation of apical efflux transporters. Panobinostat is a BCS Class II drug with high permeability and low solubility [31]. Despite high permeability, BCS class II drugs are thought to be significantly impacted by p-glycoprotein efflux, as the low solubility in the luminal space leads to concentrations insufficient to for saturating efflux transporters. It is unknown whether the impact of this classification applies to CNS membrane permeability and CNS efflux; however, our data would suggest panobinostat achieves significant CNS concentrations despite its status as a P-gp substrate.
One other in vivo study has demonstrated adequate concentrations of panobinostat in the brain [3]. Concentrations of 70 ± 10 nM and 550 ± 51 nM were identified in the cerebral cortex and brainstem tumor tissue, respectively. The cerebral cortex concentrations are consistent with the results of our study. Hennrika et al. identified concentrations in the brainstem more than 7 times that in the cerebral cortex. This further strengthens the rationale for use of panobinostat in DIPG, a tumor located in the brainstem. This study differs from ours in the method of administration of panobinostat. We administered the drug via IV, while Hennrika et al. used intraperitoneal (IP) injection for administration [3]. In addition, the aforementioned study evaluated panobinostat concentrations at a single time-point (4 h) after administration of the drug. Our study evaluated the concentration of drug at several time points, allowing for the development of a full pharmacokinetic profile and optimal pharmacokinetic modeling. We demonstrated that panobinostat maintains adequate concentrations in the brain and plasma several hours after administration.
Our study also differs from other pre-clinical and clinical models that have measured drug concentration in the cerebrospinal fluid (CSF) [15–18]. We evaluated concentration of drug in the brain tissue to more accurately reflect the site of tumor development. To exert anti-tumor activity in a brain tumor, the drug must reach adequate concentrations in the brain tissue rather than the CSF. Prior studies have likely demonstrated negligible concentrations of panobinostat in the CSF due to the drug’s lipophilicity. This is supported by other in vivo models that have demonstrated higher concentrations of panobinostat in xenograft, intestine, bladder, and heart tissue than in plasma with both IP and IV administration [32]. The pharmacokinetic data generated in this study also match scaling assumptions from other species. Data submitted to regulatory agencies report the volume of distribution of panobinostat to be 42 L/kg and 40.2 L/kg in the dog and rat, which is comparable to the 53.8 L/kg computed in this study [31]. Similarly, a clearance of 3.3 L/kg•h and 22.1 L/kg•h was observed in the dog and rat and is predicted to be 55.1 L/kg•h in the mouse based on body surface area scaling, which matches our estimate of 58.0 L/kg•h. These parameter estimates give credibility to our assay and analysis methods.
A limitation of our study is we measured total drug concentrations (bound and unbound) in the plasma and brain. It is thought that only unbound drug can pass into the CNS (given the large molecular weight of plasma proteins); however, this also does not take into account any disruption of the blood–brain barrier in the case of inflammation and tumor invasion. Because only unbound drug is thought to enter the CNS, it is possible we are underestimating the preferential distribution of panobinostat into the CNS compared to plasma with our model. However, it must be noted that the protein binding of panobinostat is estimated to be lower in murine plasma (approximately 60%) compared to human plasma (approximately 75–90%) [31, 33]. Nonetheless, it is reassuring that despite only 40% of the drug being unbound in the initial plasma compartment and able to cross the CNS, significantly more total drug was found in the CNS compartment compared to plasma, indicating preferential distribution to the desired targets in CNS tissues.
Our data support the use of panobinostat for brain tumors harboring histone mutations. Several phase I studies have evaluated panobinostat in CNS tumors [8, 34]. Wood et al. administered panobinostat to 9 pediatric patients with refractory solid tumors, including 4 patients with CNS tumors, with acceptable tolerability [8]. No patient had an objective response, but 3 patients had stable disease. A phase I study in adults evaluated panobinostat in combination with bevacizumab in twelve patients with high-grade gliomas [34]. Three patients achieved a partial response, and 6 patients had stable disease, resulting in a median progression free survival (PFS) of 4.3 months.
Several members of our institutions have established the Central Nervous System Targeted Agent Prediction Tool (CNS-TAP) to aid in selection of targeted agents for pediatric brain tumors [35]. The CNS-TAP Tool scores targeted agents based on several factors, including pre-clinical and clinical data, BBB penetration, and patient-specific genetic information to predict the agents most likely to cross the BBB and exert anti-tumor activity. However, not all agents included in the tool have information on BBB penetration, which is a limitation of its use in clinical practice. The results of this study will be used to update the CNS-TAP Tool to better predict the utility of panobinostat in specific patient cases. This model could be repeated with other targeted therapies to expand therapeutic options for the treatment of both pediatric and adult brain tumors.
Panobinostat has also been evaluated in a variety of other cancers, many with potential to metastasize to the brain [36–44]. Thus, it would be important to identify if panobinostat has activity against these diseases. Acknowledging the limitations of possible protein binding mentioned previously, at the concentrations identified in our study, panobinostat would be expected to have activity against a number of malignancies and conditions where the IC-50 values are in the low nanomolar range [36–45]. Panobinostat has also been identified as a potential therapeutic option in neurodegenerative and neuromuscular disorders, in which BBB penetration may be pertinent [46, 47]. Given the preferential distribution to CNS tissue, panobinostat should be further evaluated in in vitro and in vivo models for these disease states. Additionally, the clinical efficacy of panobinostat in pediatric and adult brain tumors and other cancers should be continually assessed through larger clinical trials.
Panobinostat has shown promise in vitro in pediatric brain tumor cell lines, particularly DIPGs with histone mutations. Current data and pharmacologic evidence has demonstrated varying probability of panobinostat’s ability to cross the blood–brain barrier. Our study suggests that panobinostat reaches adequate concentrations in brain tissue to likely exceed the IC-50 for DIPG and other malignancies with IC-50 values in the low nanomolar range. Panobinostat is a viable option for the treatment of pediatric brain tumors and should be evaluated further to assess clinical efficacy.
Supplementary Material
Acknowledgements
This study was funded with a grant awarded by the Hematology/Oncology Pharmacy Association (HOPA).
Funding
This study was funded with a grant awarded by the Hematology/Oncology Pharmacy Association (HOPA).
Footnotes
Conflict of interest All authors declare no conflicts of interest related to this manuscript.
Availability of data and material Data are available upon request to the corresponding author via email.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00280-021-04313-2.
References
- 1.Chessum N, Jones K, Pasqua E, Tucker M (2015) Recent advances in cancer therapeutics. Prog Med Chem 54:1–63 [DOI] [PubMed] [Google Scholar]
- 2.Atadja P (2009) Development of the pan-DAC inhibitor panobinostat (LBH589): successes and challenges. Cancer Lett 280:233–241 [DOI] [PubMed] [Google Scholar]
- 3.Hennika T, Guo H, Olaciregui HG, Barton KL, Ehteda A, Chitranjan A et al. (2017) Pre-clinical study of panobinostat in xenograft and genetically engineered murine diffuse intrinsic pontine glioma models. PLOS One 12(1):e0169485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hull EE, Montgomery MR, Leyva KJ (2016) HDAC inhibitors as epigenetic regulators of the immune system: imparcts on cancer therapy and inflammatory disease. Biomed Res Int 10.1155/2016/8797206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milazzo G, Mercatelli D, Muzio GD, Triboli L, De Rosa P, Perini G et al. (2020) Histone deacetylases (HDACs): evolution, specificity, role in transcriptional complexes, and pharmacological actionability. Genes 11(5):556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richardson PG, Schlossman RL, Alsina M, Weber DM, Coutre SE, Gasparetto C et al. (2013) PANORAMA 2: panobinostat in combination with bortezomib and dexamethasone in patients with relapsed and bortezomib-refractory myeloma. Blood 122(14):2331–2337 [DOI] [PubMed] [Google Scholar]
- 7.San-Miguel JF, Hungia VT, Yoon S, Beksac M, Dimopoulos MA, Elghandour A et al. (2014) Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicenter, randomized, double-blind phase 3 trial. Lancet Oncol 15:1195–1206 [DOI] [PubMed] [Google Scholar]
- 8.Wood PJ, Strong R, McArthur GA, Michael M, Algar E, Muscat AN et al. (2018) A phase I study of panobinostat in pediatric patients with refractory solid tumors, including CNS tumors. Cancer Chemother Pharmacol 82:493–503 [DOI] [PubMed] [Google Scholar]
- 9.Siegel RL, Miller KD (2020) Jemal A (2020) Cancer statistics. Ca Cancer J Clin 70(1):7–30 [DOI] [PubMed] [Google Scholar]
- 10.Braunstein S, Raleigh D, Bindra R, Mueller S, Haas-Kogan D (2017) Pediatric high-grade glioma: current and molecular landscape and therapeutic approaches. J Nerooncol 134:541–549 [DOI] [PubMed] [Google Scholar]
- 11.Mackay A, Burford A, Carvalho D, Izquierdo E, Fazal-Salom J, Taylor KR et al. (2017) Integrated molecular meta-analysis of 1,000 pediatric high-grade and diffuse intrinsic pontine gliomas. Cancer Cell 32:520–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grasso CS, Tang Y, Truffaux N, Berlow NE, Liu L, Debily M et al. (2015) Functionally-defined therapeutic targets in diffuse intrinsic pontine glioma. Nat Med 21(6):555–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (1997) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Delivery Rev 23:3–25 [DOI] [PubMed] [Google Scholar]
- 14.Marini BL, Benitez LL, Zureick AH, Sallou R, Gauthier AC, Brown J et al. (2017) Blood brain barrier-adapted precision medicine therapy for pediatric brain tumors. Transl Res 188:27.e1–27.e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rasmussen TA, Tolstrup M, Møller HJ, Brinkmann CR, Olesen R, Erikstrup C et al. (2015) Activation of latent human immunodeficiency virus by the histone deacetylase inhibitor panobinostat: a pilot study to assess effects on the central nervous system. Open Forum Infect Dis 2(1):ofv037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guntner AS, Peyrl A, Mayr L, Englinger B, Berger W, Slavc I et al. (2020) Cerebrospinal fluid penetration of targeted therapeutics in pediatric brain tumor patients. Acta Neuropathol Commun 8:e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldberg J, Sulis ML, Bender J, Jeha S, Gardner R, Pollard J et al. (2020) A phase I study of panobinostat in children with relapsed and refractory hematologic malignancies. Pediatr Hematol Oncol. 10.1080/08880018.2020.1752869 [DOI] [PubMed] [Google Scholar]
- 18.Rodgers LT, Lester McCully CM, Odabas A, Cruz R, Peer CJ, Figg WD et al. (2020) Characterizing the pharmacokinetics of panobinostat in a non-human primate model for the treatment of diffuse intrinsic pontine glioma. Cancer Chemoth Pharmacol 85(4):827–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ballinger MB, Baneux PJ, Barthold SW, Cork LC, Hau J, Huerkamp MJ, et al. (2011) Guide for the care and use of laboratory animals, 8th edn. Washington, D.C. [Google Scholar]
- 20.Karol SE, Cooper TM, Mead PE, Crews KR, Panetta JC, Alexander TB et al. (2020) Safety, pharmacokinetics, and pharmacodynamics of panobinostat in children, adolescents, and young adults with relapsed acute myeloid leukemia. Cancer 126:4800–4805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barber TW, Brockway JA, Higgins LS (1970) The density of tissues in and about the head. Acta Neurol 46:85–92 [DOI] [PubMed] [Google Scholar]
- 22.DeAngelo DJ, Walker AR, Schlenk RF, Sierra J, Medeiros BC, Ocio EM et al. (2019) Safety and efficacy of oral panobinostat plus chemotherapy in patients ages 65 years or younger with high-risk acute myeloid leukemia. Leuk Res. 10.1016/.j.leukres.2019.106197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Veggel M, Westerman E, Hamberg P (2018) Clinical pharmacokinetics and pharmacodynamics of panobinostat. Clin Pharmacokinet 57(1):21–29 [DOI] [PubMed] [Google Scholar]
- 24.Tengvar C, Forssen M, Hultstrom D, Olsson Y, Pertoft H, Pettersson A (1982) Use of Percoll density gradients for determination of specific gravity in cerebral cortex and white matter under normal conditions and in experimental cytotoxic brain edema. Acta Neuropathol 57:143–150 [DOI] [PubMed] [Google Scholar]
- 25.Daina A, Zoete V (2016) A BOILED-Egg to predict gastrointestinal absorption and brain penetration of small molecules. ChemMedChem 11(11):1117–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miklja Z, Mullan B, Siada R, Stallard S, Yadav VN, Bruzek A et al. (2019) The effect of everolimus on CNS penetration and efficacy of dasatinib in the treatment of PDGFRA-driven glioma. J Clin Oncol 37(15_suppl):e13508 [Google Scholar]
- 27.Minocha M, Khurana V, Qin B, Pal D, Mitra AK (2012) Coadministration strategy to enhance brain accumulation of vandetanib by modulating P-glypoprotein (P-gp/Abcb1) and breast cancer resistance protein (Bcrp1/Abcg2) mediated efflux with m-TOR inhibitors. Int J Pharm 454(1–2):306–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varma MV, Sateesh K, Panchagnula R (2005) Functional role of P-glycoprotein in limiting intestinal absorption of drugs: contribution of passive permeability to P-glycoprotein mediated efflux transport. Mol Pharm 2(1):12–21 [DOI] [PubMed] [Google Scholar]
- 29.Dahan A, Amidon GL (2009) Segmental dependent transport of low permeability compounds along the small intestine due to P-glycoprotein: the role of efflux transport in the oral absorption of BCS class III drugs. Mol Pharm 6(1):19–28 [DOI] [PubMed] [Google Scholar]
- 30.Porat D, Dahan A (2018) Active intestinal drug absorption and the solubility-permeability interplay. Int J Pharm 537(1–2):84–93 [DOI] [PubMed] [Google Scholar]
- 31.Place EJ, Ringgold K (2014) Pharmacology/toxicology NDA review and evaluation of panobinostat. Center for drug evaluation and research. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/205353Orig1s000PharmR.pdf. Accessed 15 Dec 2020
- 32.Groselj B, Ruan J, Scott H, Gorrill J, Nicholson J, Kelly J et al. (2018) Radiosensitization in vivo by histone deacetylase inhibition with no increase in early normal tissue radiation toxicity. Mol Cancer Ther 17(2):381–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grillo J, Habtemariam B, Ma L, Mehrotra N (2014) Office of clinical pharmacology review addendum. Center for drug evaluation and research. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/205353Orig1s000PharmR.pdf. Accessed 10 May 2021
- 34.Drappatz J, Lee EQ, Hammond S, Grimm SA, Norden AD, Beroukhim R et al. (2012) Phase I study of panobinostat in combination with bevacizumab for recurrent high-grade glioma. J Neurooncol 107:133–138 [DOI] [PubMed] [Google Scholar]
- 35.Linzey JR, Marini BL, Pasternak A, Smith C, Mikja Z, Zhao L et al. (2018) Development of the CNS TAP tool for the selection of precision medicine therapies in neuro-oncology. J Neurooncol 137(1):155–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu X, Gomex-Pinillos A, Liu X, Johnson EM, Ferrari AC (2010) Induction of bicalutamide sensitivity in prostate cancer cells by an epigenetic pura-mediated decreased in androgen receptor levels. Prostate 70:179–189 [DOI] [PubMed] [Google Scholar]
- 37.Wang L, Syn NL, Subhash VV, Any Y, Thuya WL, Cheow ESH et al. (2018) Pan-HDAC inhibition by panobinostat mediates chemosensitization to carboplatin in non-small cell lung cancer via attenuation of EGFR signaling. Cancer Lett 417:152–160 [DOI] [PubMed] [Google Scholar]
- 38.Maiso P, Colado E, Ocio EM, Garayoa M, Martin J, Atadja P et al. (2009) The synergy of panobinostat plus doxorubicin in acute myeloid leukemia suggests a role for HDAC inhibitors in the control of DNA repair. Leukemia 23(12):2265–2274 [DOI] [PubMed] [Google Scholar]
- 39.Scuto A, Kirschbaum M, Kowolik C, Kretzner L, Juhasz A, Atadja P et al. (2008) The novel histone deacetylase inhibitor, LBH589, induces expression of DNA damage response genes and apoptosis in Ph- acute lymphoblastic leukemia cells. Blood 111(10):5093–5100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen S, Ye J, Kijima I, Evans D (2010) The HDAC inhibitor LBH589 (panobinostat) is an inhibitory modulator of aromatase gene expression. PNAS 107(24):11032–11037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saitoh Y, Bureta C, Sasaki H, Nagano S, Maeda S, Furukawa T et al. (2019) The histone deacetylase inhibitor LBH589 inhibits undifferendtiated pleomorphic sarcoma growth via downregulation of FOS-like antigen 1. Mol Carcinogen 58:234–246 [DOI] [PubMed] [Google Scholar]
- 42.Choi SA, Lee C, Kwak PA, Park C, Wang K, Phi JH et al. (2019) Histone deacetylase inhibitor panobinostat potentiates the anti-cancer effect of mesenchymal stem cell-based sTRAIL gene therapy against malignant glioma. Cancer Lett 442:161–169 [DOI] [PubMed] [Google Scholar]
- 43.Bluethner T, Niederhagen M, Caca K, Serr F, Witzigmann H, Moebius C et al. (2007) Inhibition of histone deacetylase for the treatment of biliary tract cancer: a new effective pharmacological approach. World J Gastroenterol 13(35):4761–4770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maiso P, Carvajal-Vergara X, Ocio EM, Lopez-Perez R, Mateo G, Gutierrez N et al. (2006) The histone deacetylase inhibitor LDH589 is a potent antimyeloma agent that overcomes drug resistance. Cancer Res 66(11):5781–5789 [DOI] [PubMed] [Google Scholar]
- 45.Chua M, Arnold MSJ, Xu W, Lancelot J, Lamotte S, Spath GF et al. (2017) Effect of clinically approved HDAC inhibitors on Pasmodium, Leishmania, and Schistosoma parasite growth. Int J Parasitol Drugs Drug Resist 7(1):42–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh AK, Halder-sinha S, Clement JP, Kundu TK (2018) Epigenetic modulation by small molecule compounds for neurodegenerative disorders. Pharmacol Res 132:135–148 [DOI] [PubMed] [Google Scholar]
- 47.Pagliarini V, Guerra M, Di Rosa V, Compagnucci C, Sette C (2020) Combined treatment with the histone deacetylase inhibitor LBH589 and a splice-switch antisense oligonucleotide enhances SMN2 splicing and SMN expression in spinal muscular atrophy cells. J Neurochem 153(2):264–275 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.