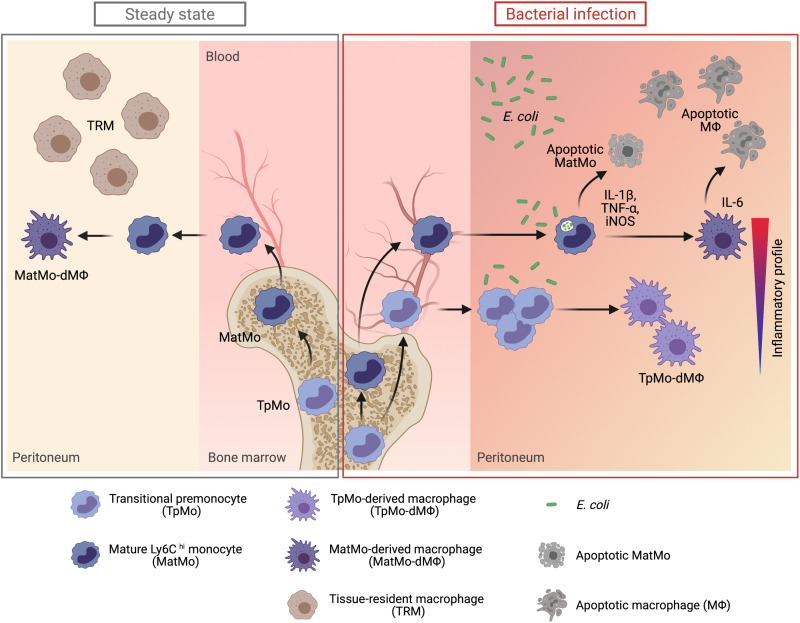
Fig. 8. The mobilization of TpMos into the periphery allows a diversification of monocytic roles to protect the host against bacterial infections.
During steady state, TpMos are immobilized in the BM where they serve as a reservoir of precursor cells to replenish the pool of MatMos in the circulation. Consequently, the replenishment of TRMs in the periphery are carried out solely by MatMos, giving rise to MatMo-dMΦs. However, upon bacterial infection, TRMs undergo rapid cellular death, which predisposes the host toward morbidity from an empty macrophage niche and the ensuing bacterial burden. This scenario results in competing demands placed on MatMos, which must now fulfil both antibacterial effector functions and macrophage replenishment roles simultaneously. To circumvent this challenge, TpMos are mobilized from the BM into the circulation to serve as the main source of macrophage replenishment when MatMos are vulnerable toward bacteria-induced cell death after the exhaustion of their effector functions. Furthermore, TpMos and their derived macrophages provided protection against sepsis by balancing the highly proinflammatory cytokine response of MatMos that contribute toward the cytokine storm in sepsis. Together, our findings highlight a specialization of monocytic roles against bacteria invasion through the mobilization of a monocyte precursor, which may be an evolutionary designed mechanism to balance the competing demands placed on the immune system in their arms race against pathogens. (Figure created with BioRender.com.)