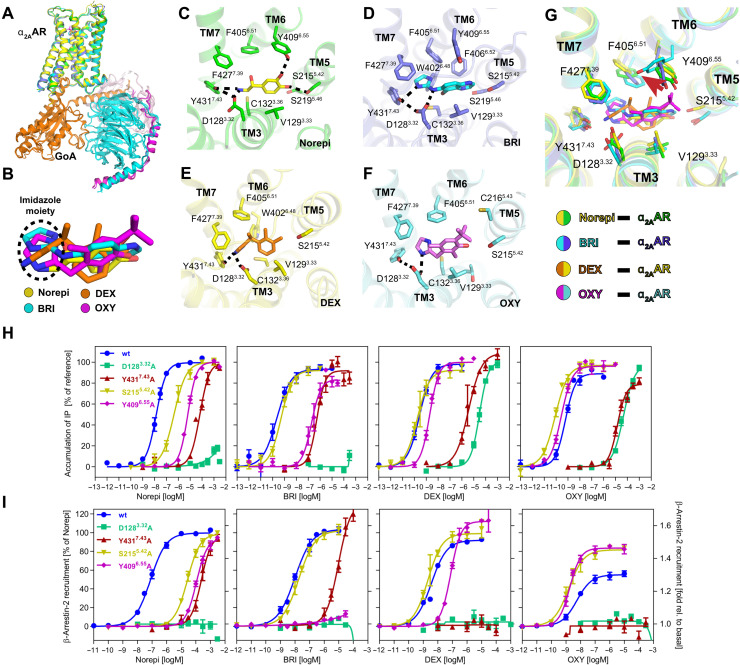
Fig. 3. Orthosteric binding pocket of active α2AAR bound to different agonists.
(A) Alignment of four structures of α2AAR signaling complexes. (B) Superposition of Norepi, BRI, DEX, and OXY from cryo-EM structures. The imidazole moiety is highlighted by the dashed circle. (C to F) Detailed interactions of Norepi (C), BRI (D), DEX (E), and OXY (F) with α2AAR. Residues within 4 Å of agonist are shown in sticks. The polar interactions are indicated by black dashed lines. (G) Superposition of the α2AAR orthosteric binding pocket residues bound to four different agonists. (H and I) Concentration-response curves of different agonists for G protein activation (H) and β-arrestin-2 recruitment (I) for wild-type (wt) α2AAR and receptor mutants D1283.32A, Y4317.43A, S2155.42A, and Y4096.55A, respectively. Except for the activation of D1283.32A, which is normalized to DEX for G protein activation and relative to basal for arrestin recruitment, receptor activation is shown relative to the maximum effect of Norepi. Data are presented as means ± SEM of 3 to 11 independent experiments with repeats in duplicate.