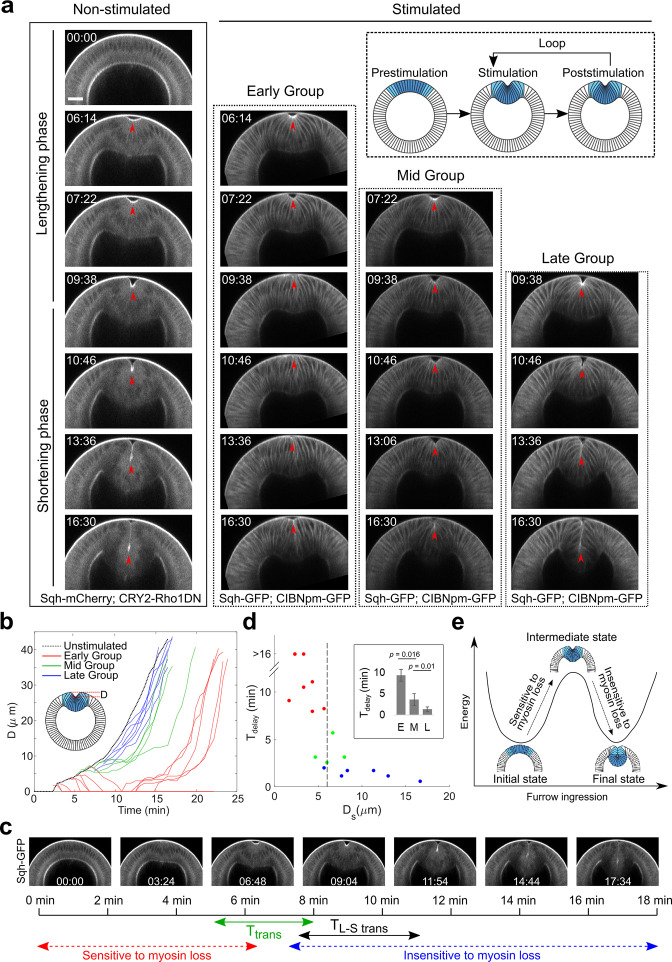
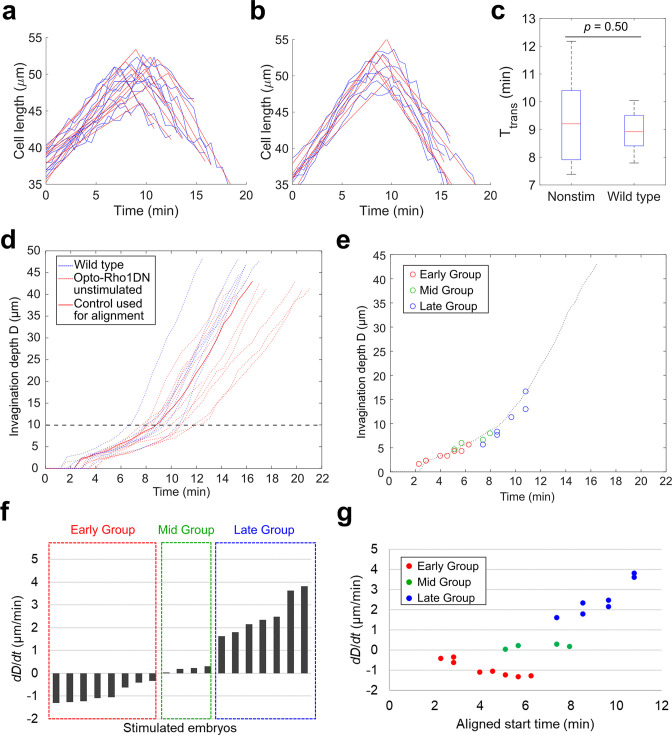
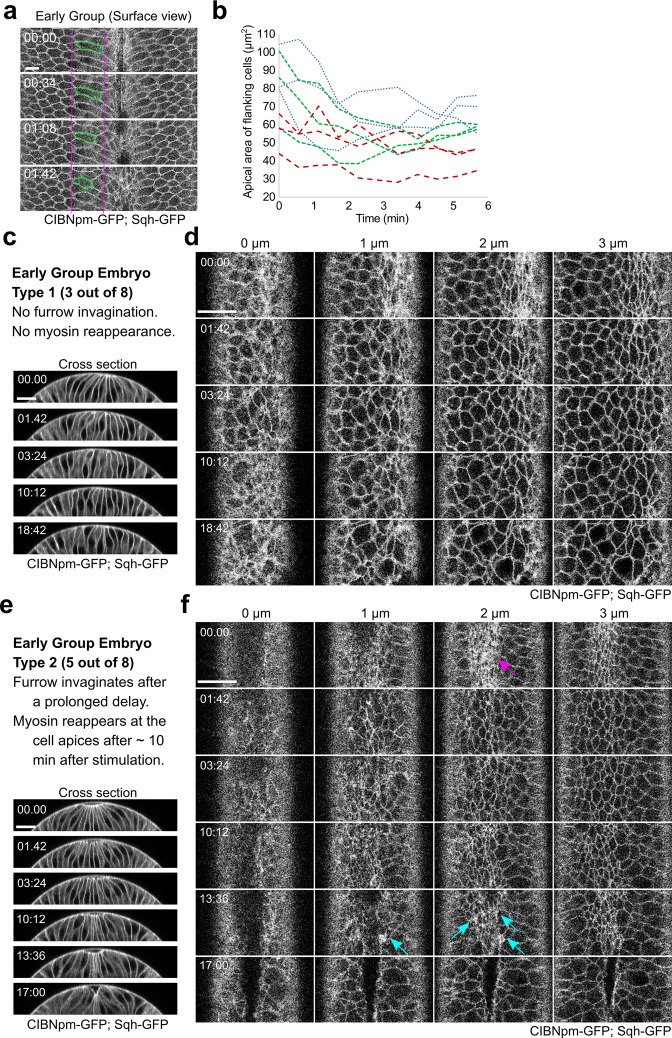
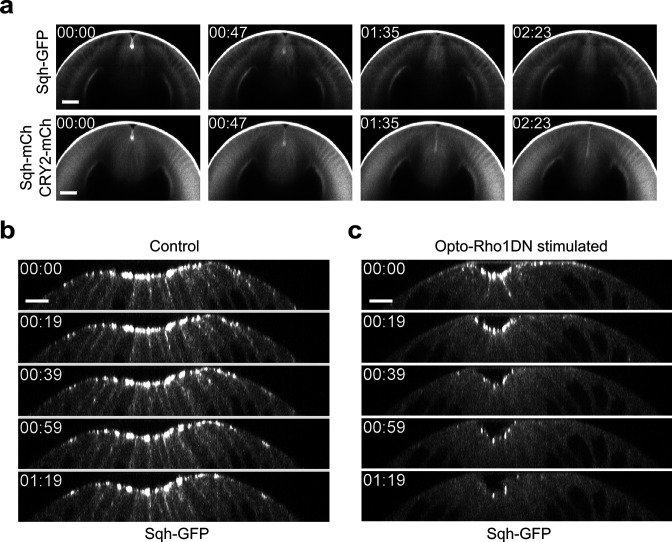
Figure 4. Acute inhibition of actomyosin contractility results in stage-dependent response during ventral furrow formation.
(a) Still images from multiphoton movies showing different tissue responses to acute loss of myosin contractility during ventral furrow formation. Early Group, Mid Group, and Late Group embryos (N=8, 4, and 6 embryos, respectively) are defined based on their immediate response to myosin inhibition. For stimulated embryos, the first frame corresponds to the time point immediately after stimulation. The inset depicts the stimulation and imaging protocol. Arrowheads indicate the apex of the ventral most cells. (b) Time evolution of the invagination depth ‘D’ for the stimulated embryos and a representative unstimulated control embryo. For stimulated embryos, all movies were aligned in time to the representative control embryo based on furrow morphology at the time of stimulation. (c) Relationship between the transition phase for sensitivity to myosin inhibition (Ttrans) and lengthening-shortening transition (TL-S trans). (d) Scatter plot showing the relation between invagination depth at the time of stimulation (Ds) and the delay time (Tdelay) in furrow invagination compared to the representative control embryo. Tdelay is highly sensitive to Ds, with a switch-like change at Ds~6 μm (dashed line). Inset: Average Tdelay in Early (E, n=5 embryos that invaginated), Mid (M, n=4), and Late (L, n=6) Group embryos. Statistical comparisons were performed using two-sided Wilcoxon rank-sum test. (e) Cartoon depicting mechanical bistability of the mesoderm during gastrulation. Both the initial, pre-constriction state and the final, fully invaginated state are stable. During gastrulation, actomyosin contractility is critical for bringing the system from the initial state to an intermediate, transitional state, whereas the subsequent transition to the final state can occur independent of myosin contractility.