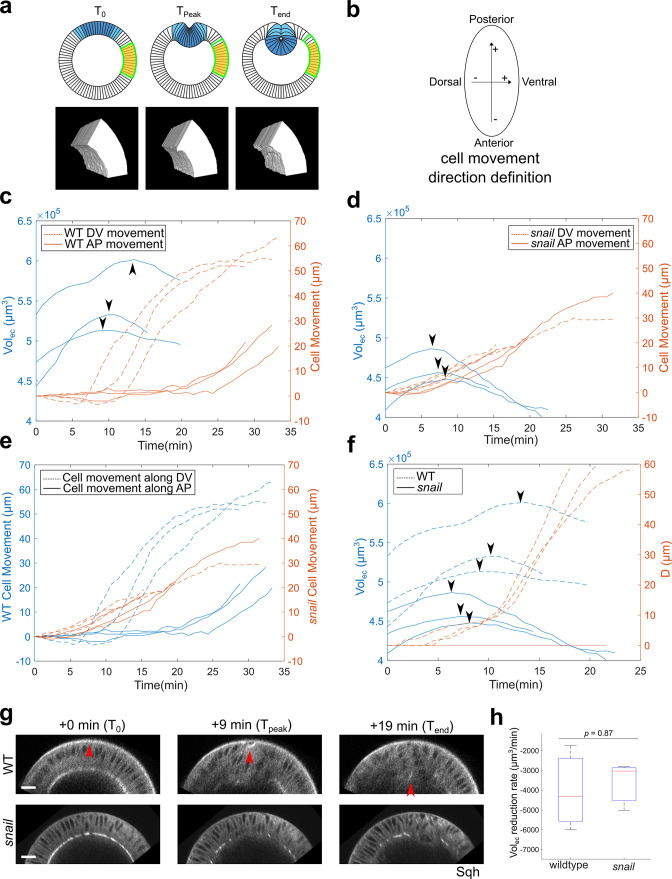
Figure 8. The lateral ectoderm undergoes apical-basal shortening during gastrulation independently of ventral furrow formation.
(a) Top: cartoon showing a cross-section view of an embryo. Blue: mesoderm. Yellow: segmented ectoderm region (ROI). Green: the same group of ectoderm cells. The ROI (in 3D) covers the lateral ectodermal region that is 60°–120° away from the ventral midline and 75-μm long along the AP axis. The change in the volume of ROI (Volec) is used as a readout for change in average tissue thickness. Bottom: representative segmented 3D views showing the ectoderm at the onset of apical constriction (T0), reaches the thickest point (Tpeak) and at the last time frame of ventral furrow formation that is reliably segmented (Tend). (b) Definition for the direction of cell movement used in (c–f). (c, d) Change in Volec over time and cell movement along A-P and D-V axis in wild-type (c) and snail mutant embryos (d). Arrowheads indicate the start of ectoderm shortening. (e) Cell movement along A-P and D-V axis in wild-type and snail mutants are replotted together for better comparison. (f) Change in Volec and invagination depth D over time in wild-type and snail mutant embryos. Arrowheads indicate the start of ectoderm shortening. (g) Representative cross-section views showing the wild-type and snail mutant embryos at T0, Tpeak, and Tend, respectively. Arrowheads indicate the apex of the mid constricting cells. N=3 embryos for each genotype. Scale bars: 20 μm. (h) Comparison of the ectoderm volume reduction rate between the wild-type and snail mutant embryos. The descending part of the volume curve was fitted into a straight line to calculate the rate of volume reduction.