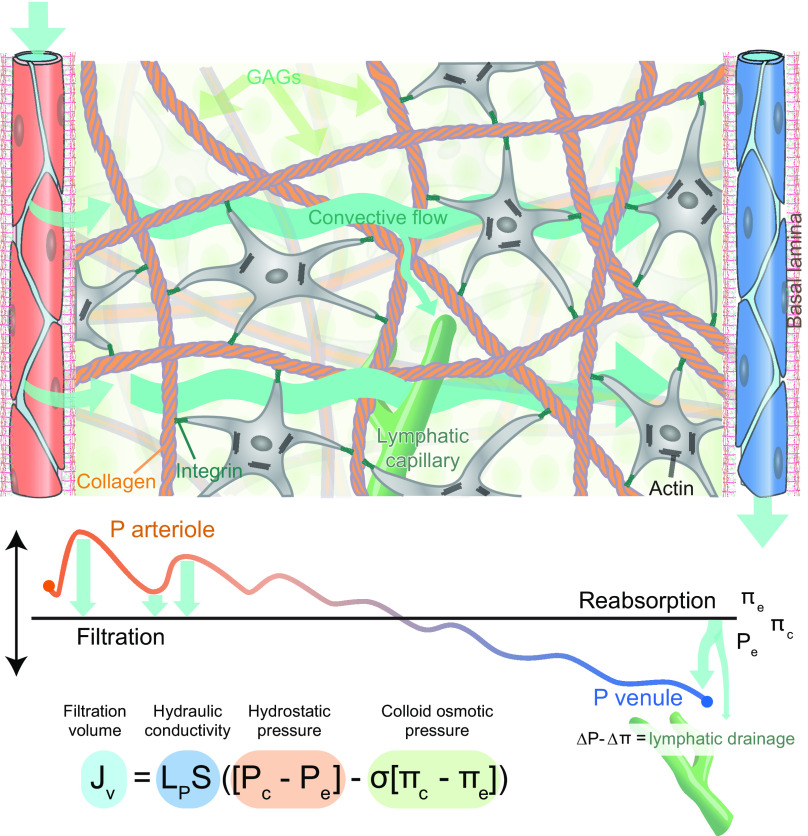
FIGURE 13.
Starling’s principle of hydrostatic pressure driving convective fluid flow in peripheral tissue. Capillaries in peripheral tissues are fenestrated and covered by a basal lamina. This arrangement allows an ultrafiltrate of plasma containing relatively low molecular weight solutes such as glucose and globular proteins to flow into the tissue, driven by hydrostatic pressure (Pc). Pc oscillates with the cardiac cycle, exhibiting higher pressures during systole and lower pressures during diastole, which drive temporally changing filtration volumes rather than continuous inflow. The directional flow of fluid and its solutes away from the arterial end of the capillary bed is supported by the colloid osmotic (oncotic) pressure (πc) absorbing fluid in the venous end of the capillary bed. The remaining fluid and solutes (ΔP − Δπ) are transported out of the tissue by lymphatic capillaries (green). The ultrafiltrate of plasma do not experience unrestricted flow within the tissue, because of interference from cells and the fibrous elements of the extracellular matrix proteins (mostly collagen). Fluid flows primarily within the gelatinous pockets of the proteoglycans/hyaluronan containing glycosaminoglycan (GAGs.) When the hydration of GAGs is high, the hydraulic resistance is low, resulting in faster and more substantial fluid transport. On the other hand, dehydration of proteoglycans/hyaluronan will increase the hydraulic resistance and thereby reduce the inflow of the plasma ultrafiltrate. The relative contribution of the endothelial cell glycocalyx and hydration of GAG in regulating the rate of plasma ultrafiltration has not been determined. The tension within the tissue is created by the fibrillary network of collagen connected to the cytoskeleton by integrin receptors and serves to limit the extent of GAG hydration.