FIGURE 15.
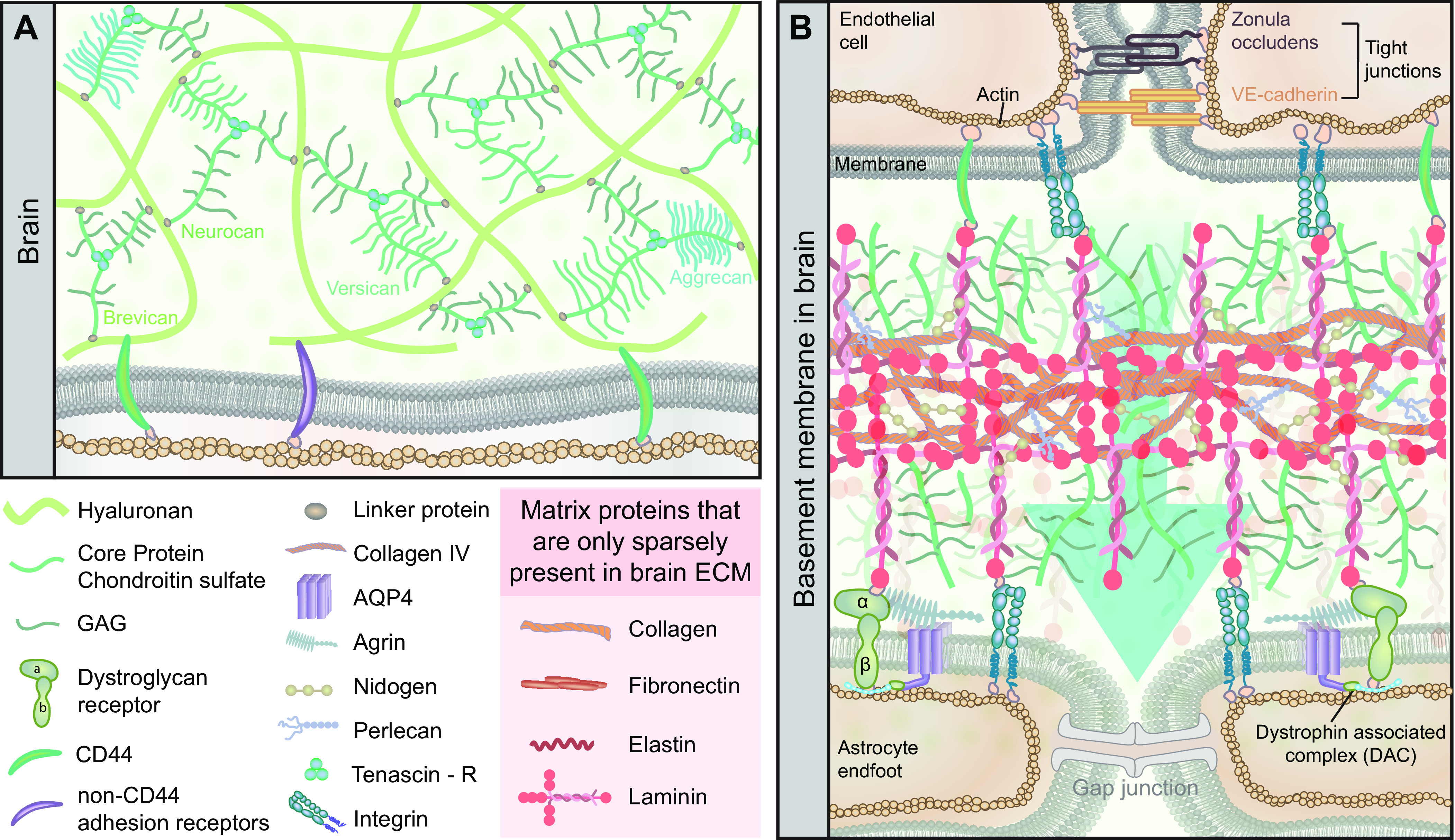
Brain extracellular matrix. A: the extracellular matrix (ECM) in the brain is distinct from that in the periphery with respect to its near absence of protein components, being practically devoid of collagen, except collagen IV in the basement membranes, fibronectin, elastin, and laminins. The brain extracellular matrix is primarily composed of glycosaminoglycans (GAGs), mostly hyaluronan and chondroitin sulfate proteoglycans (CSPGs). CSPGs have a wide array of isoforms termed lecticans, including aggrecan, neurocan, brevican, and versican, which are linked together in a matrix via tenascin R. The actin cytoskeleton of cells in the neuropil bind to the CSPGs in the extracellular matrix via CD44 and other non-CD44 adhesion receptors. B: the vascular basement membrane is one of the few locations in the CNS where the extracellular matrix is fibrous, being composed of structural proteins similar to those found in peripheral basement membranes. In particular, the vascular basement membrane is composed of a 3-dimensional matrix of collagen IV and laminins. The isoform of laminin varies between different segments of the vascular network. The protein-rich vascular basement membrane matrix is stabilized by cross-linked nidogen and perlecan. Brain endothelial cells are interconnected via cadherin and the zonula occludens tight junctions, which together form the blood-brain barrier (BBB). The BBB inhibits the free entry of plasma water and many of its solutes from the vasculature. Endothelial cells attach onto the basement membrane via CD44 and integrin receptors in an interaction resulting in the upregulation of tight junction proteins, thus conferring to them their barrier properties. Astrocyte endfeet connected by gap junctions line the opposite side of the basement membrane and attach to the basement membrane via dystroglycan and integrin receptors. The dystroglycan receptor is composed of α- and β-subunits. The attachment of the α-dystroglycan to agrin in the basement membrane, together with the dystrophin-associated protein complex (i.e., dystrophin, dystrobrevin, and α-syntrophin), stabilize the localization of orthogonal arrays of intramembranous proteins composed primarily of aquaporin-4 (AQP4) water channels.
