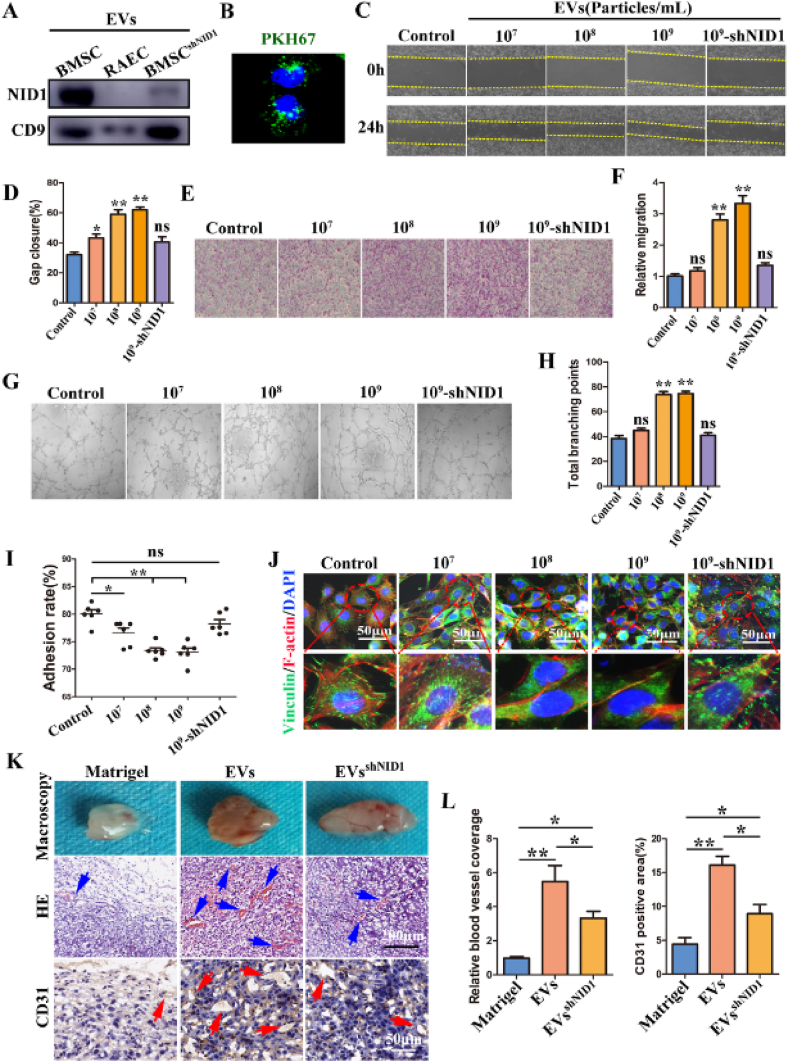
Fig. 3.
EV-NID1 significantly enhances the migration and vascularization capabilities of RAECs by regulating cell adhesion. (A) The abundance of NID1 in EVs derived from BMSC, RAEC and BMSCshNID1 was determined by WB. (B) PKH67-labeled EVs were internalized by RAECs. (C and D) EVs derived from BMSCs were incubated with RAECs for 48 h, the effect of EVs on the wound healing ability of RAECs was evaluated by scratch experiment. (E and F) Transwell migration assay. (G and H)Tubule formation in Matrigel, and the total branching points were compared statistically. (I)The adhesion rate of RAECs. (J) Immunofluorescence staining images of vinculin and F-actin. (K) The EVs or EVsshNID1 with the concentration of 109 particles/mL (PBS as control) were loaded into matrigel and subcutaneously implanted into nude mice. The angiogenesis in matrigel was evaluated at 14 days post operation. The blue arrow represents neovascularization, and the red arrow represents CD31+ blood vessels. (L) Comparison of relative vascular coverage and CD31 positive areas in matrigel. Data were represented as the mean ± SD.*p < 0.05; **p < 0.01; ns, not significant from Student's t-test.