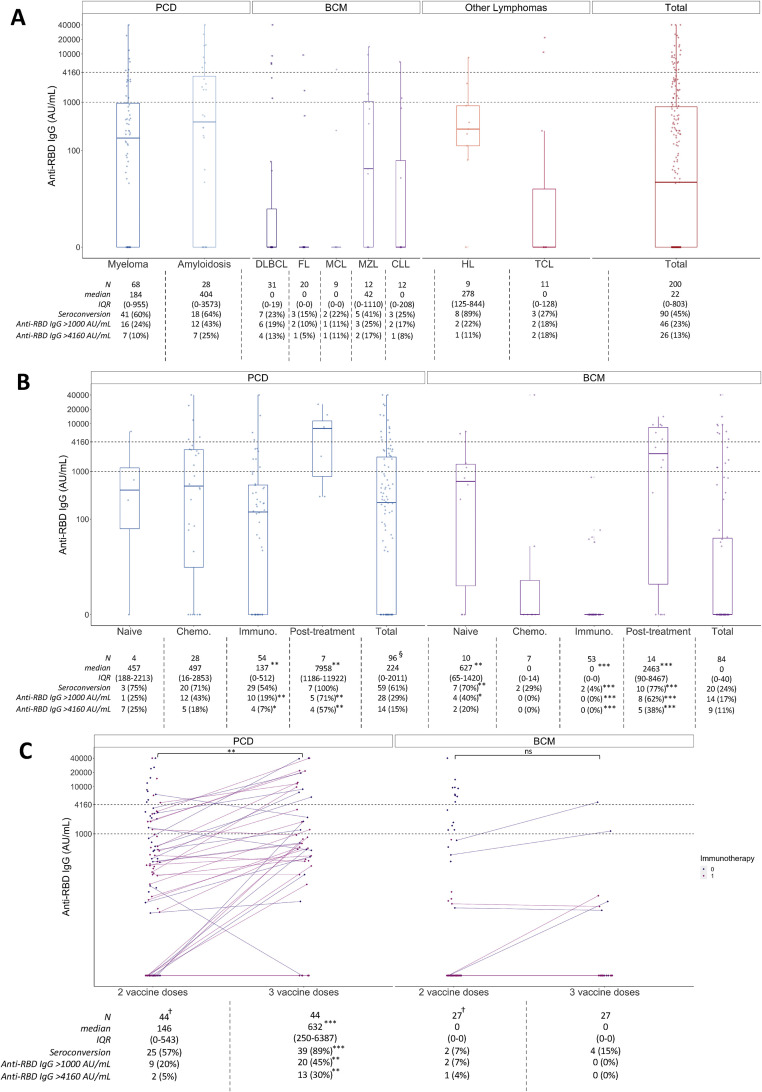
Fig. 1.
Immunogenicity of two and three mRNA vaccines doses among patients with lymphoid malignancies. Graphics display a representation of the median anti-RBD IgG concentration value (IQR) through boxplot representation. To appreciate the data dispersion, interquartile range was included in the attached tables. (a) Anti-RBD IgG concentration (AU/mL) according to underlying haematological pathology, after two mRNA vaccine doses. (b) Anti-RBD IgG concentration (AU/mL) according to main pathology groups and treatment characteristics, after two mRNA vaccine doses. “Naïve” condition represents patients who received at least one vaccine dose before initiation any treatment; “Chemotherapy” condition represents patients who only received in the previous year standard cytotoxic chemotherapy as treatment; “Immunotherapy” condition represents patients who received in the previous year either anti-CD20 or anti-CD38 therapy (accordingly to the underlying disease); “Post-treatment” condition represents patients who had their last treatment at least 12 months before vaccination. Statistical analyses were made by comparing each group to the other three. (c) IgG anti-RBD IgG concentrations (AU/mL) after two and three mRNA vaccine doses, according to main pathology groups and stratified into subgroups based on the use of immunotherapy in last 12 months (anti-CD20 or anti-CD38 accordingly to the underlying disease). CLL, chronic lymphoid leukaemia; DLBC, diffuse large B cell lymphoma; FL, follicular lymphoma; HL, Hodgkin lymphoma; MCL, mantle cell lymphoma; MZL, marginal zone lymphoma and lymphoplasmacytic lymphoma/Waldenström macroglobulinemia; TCL, T cell lymphoma. †Graphic displays the whole population vaccinated with two doses to identify the patients selected for a third vaccine dose (44 and 27 patients in PCD and BCM groups, respectively); attached table describes the population who received three vaccine doses. §Three patients affected by plasma cell disorders did not have clear or available treatment information. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ns: not statistically significant.