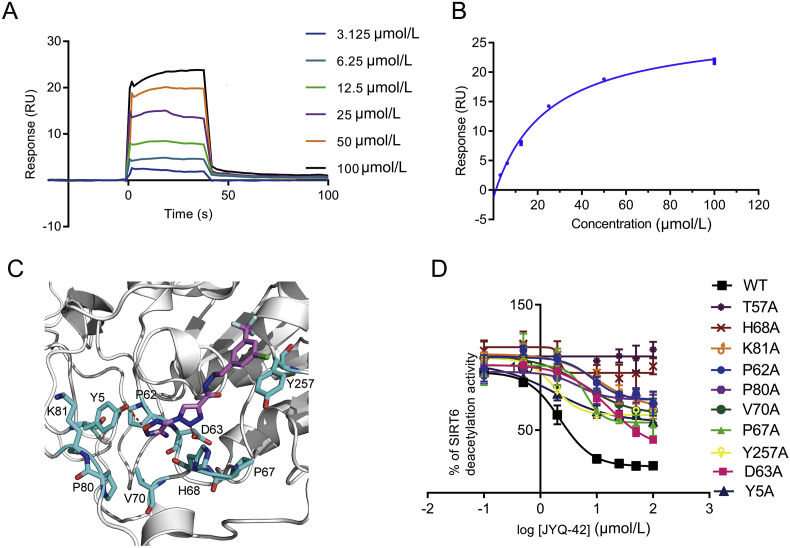
Figure 4.
JYQ-42 is an allosteric inhibitor of SIRT6. (A) The binding effect of JYQ-42 on SIRT6 was analysed by surface plasmon resonance (SPR). Three independent experiments were performed. (B) SPR binding curves fitted with steady-state model showing the binding affinity of JYQ-42. The Kd value of JYQ-42 was 22.03 ± 3.11 μmol/L, and three independent experiments were performed. (C) Molecular docking of SIRT6 with JYQ-42. The SIRT6-ADPr crystal structure (PDB ID: 3K35) was used for docking, and the best JYQ-42 binding pose with the smallest Glide/IFD score or lowest energy was chosen for further analysis. (D) Effects of allosteric-site mutations located in the JYQ-42 binding pocket on the inhibition of SIRT6 deacetylation, as determined by FDL assays. Data are presented as the mean ± SD, n = 3 wells, from three independent experiments.