Abstract
Increasing demand for wearable healthcare synergistically advances the field of electronic textiles, or e-textiles, allowing for the ambulatory monitoring of vital health signals. Despite great promises, the pragmatic deployment of e-textiles in clinical practice remains challenged due to the lack of a method in producing custom-designed e-textiles at high spatial resolution across a large area. To this end, we employ a programmable dual regime spray that enables the direct custom writing of functional nanoparticles into arbitrary fabrics at submillimeter resolution over meter scale. The resulting e-textiles retain the intrinsic fabric properties in terms of mechanical flexibility, water vapor permeability, and comfortability against multiple uses and laundry cycles. The e-textiles tightly fit various body sizes and shapes to support the high-fidelity recording of physiological and electrophysiological signals on the skin under ambulatory conditions. Pilot field tests in a remote health monitoring setting with a large animal, such as a horse, demonstrate the scalability and utility of the e-textiles beyond conventional devices. This approach will be suitable for the rapid prototyping of custom e-textiles tailored to meet various clinical needs.
Keywords: electronic textiles, dual regime spray, programmable direct patterning, ambulatory health monitoring, telehealthcare
Graphical Abstract

Horse in a custom e-textile under ambulatory conditions for remote health monitoring.
1. Introduction
Electronic textiles, or e-textiles, are of particular interest in remote health monitoring, which enables the ambulatory monitoring of health condition or disease progression on a routine or continuous basis.[1-5] E-textiles are typically formed through the additive patterning of functional nanomaterials into fabrics in a configuration to detect physiological and electrophysiological responses on the skin.[6,7] Current approaches in producing e-textiles involve using either wet spinning, biscrolling, dip coating, screen printing, inkjet printing, dip pen nanolithography, physical vacuum depositing, or weaving to overcoat fabrics with functional nanomaterials.[8-14] In these approaches, precise control in the overcoat uniformity is a key to ensure the mechanical and electrical reliability of e-textiles during use in ambulatory health monitoring.[15,16] Despite great advances, a critical need still remains to meet the following requirements at the same time: (1) large-scale batch production through a mask-free writing of functional nanomaterials at desired shape and size; (2) seamless mechanical and electrical connection of functional nanomaterials through their deep and uniform embedment into fabrics without aggregation; (3) high spatial resolution through a fine patterning of functional nanomaterials into custom sensor designs; and (4) long-term durability against multiple uses and laundry cycles. All these features are integral to the pragmatic deployment of e-textiles in clinical practice.
To this end, we introduce a unique approach that involves using a programmable dual regime spray to produce custom-designed e-textiles at high spatial resolution (at submillimeter scale) across a large area (over meter scale) beyond conventional approaches. The dual regime spray includes two separate (i.e., high- and low-speed) air flow modules on a three-axis computer numerical control (CNC) gantry.[17] This setting enables the direct custom spray of conducting nanoparticles into arbitrary fabrics at precisely controlled mass loading and penetration depth without the need of a shadow mask or dedicated vacuum equipment that is often required in current approaches.[18,19] This setting also enables the uniform seamless coating of a waterproof elastomer over the as-sprayed conducting nanoparticles as a sealing overcoat to provide physical and chemical protection against multiple uses and laundry cycles. This study uncovered the process-structure-property relationships of various e-textiles for their implementations in a remote health monitoring setting with a large animal such as a horse.
2. Results and Discussion
2.1. Dual regime spray process
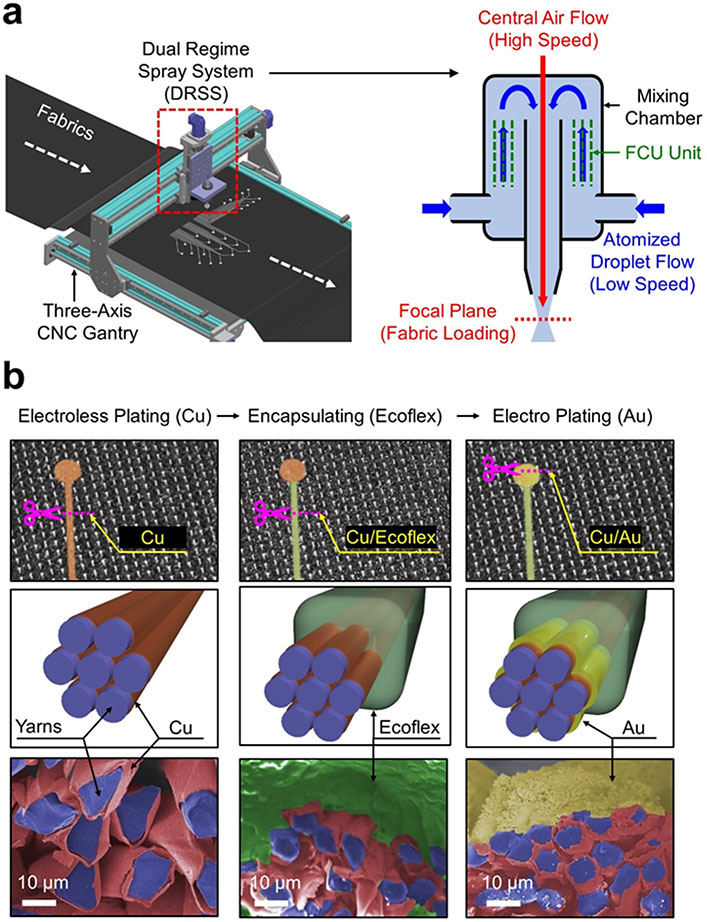
Figure 1a provides schematic illustrations of the dual regime spray process to generate e-textiles. The corresponding photographs of this setting are shown in Figure S1, Supporting Information. The dual regime spray process began by atomizing a 73 mM of silver nitrate (AgNO3) solution in a 1:15 mixture of water and ethanol by volume into fine droplets with a pneumatic atomizer (WestMed-0210; WixOne) at a pressure of 70 kPa. The atomized droplets containing AgNO3 nanoparticles were carried in a mixing chamber by a low-speed air flow (i.e., < 10 m sec−1) to minimize cyclone effects (i.e., uniform dispersion).[17] The droplets were then passed through an internal flow-conditioning unit (FCU) to sort out large droplets. At the nozzle exit, the droplets were accelerated by a high-speed (i.e., > 100 m sec−1) central air flow to remain focused at a focal plane, thereby allowing for the narrow and deep penetration of the AgNO3 nanoparticles into fabrics. Figure S2a (Supporting Information) shows the cross-sectional scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDX) images of the as-sprayed AgNO3 nanoparticles into a Lycra fabric to confirm their uniform seamless coating without aggregation. Figure S2b (Supporting Information) shows the experimental results of multiple peeling tests using a Scotch tape (3M Inc) to confirm the adhesion between the AgNO3 nanoparticles and the fabric. No alteration in the intrinsic fabric structure was noticed during or after the dual regime spray process. A real-time video of the dual regime spray process is shown in Movie S1.
Figure 1. Dual regime spray process.
(a) Schematic illustration of the dual regime spray process using a three-axis CNC gantry. (b) Schematic illustrations and colored SEM images for the subsequent overcoating of Cu, Ecoflex, and Au from the left column.
Figure 1b shows the schematic illustrations and colored SEM images of the sprayed AgNO3 nanoparticles in a Lycra fabric following subsequent overcoating of copper (Cu), Ecoflex, and gold (Au) from the left column. First, a 800 nm-thick layer of Cu was electrolessly plated over the sprayed AgNO3 nanoparticles to complete conduction paths. The electrical conductivity of the Cu overcoat increased up to 9,400 S cm−1 after 6 hours of the electroless plating, resulting in the grain size of 500 ± 37 nm on average (Figure S3, Supporting Information). For comparison, a solution of Ag nanowires (Sigma Aldrich Inc) was also sprayed into a Lycra fabric, but at least 100 times in order to obtain a similar resistance to that of the Cu overcoat (Figure S4, Supporting Information). Thereby, the material consumption and production time in spraying the Ag nanowires were substantially higher compared to the AgNO3 nanoparticles with the Cu overcoat. Next, a 60 μm-thick layer of Ecoflex was coated to seamlessly cover the conduction paths, except the areas of recording electrodes, thereby providing electrical insulation, mechanical protection, and waterproof barrier against multiple uses and laundry cycles.[20,21] Lastly, a 1.5 μm-thick layer of Au was electroplated over the exposed surface of the Cu overcoat (i.e., recording areas) at 5 V for a minute to promote biocompatibility and also prevent oxidation.[22,23] Figure S5 (Supporting Information) shows the X-ray diffraction (XRD) and EDX images of the overcoat layers to confirm the uniformity. The dual regime spray process was applicable to various types of synthetic fabrics made of cotton, polyester, and Lycra without noticeable difference in the overall coating quality (Figure S6, Supporting Information).
2.2. Optimal operational condition
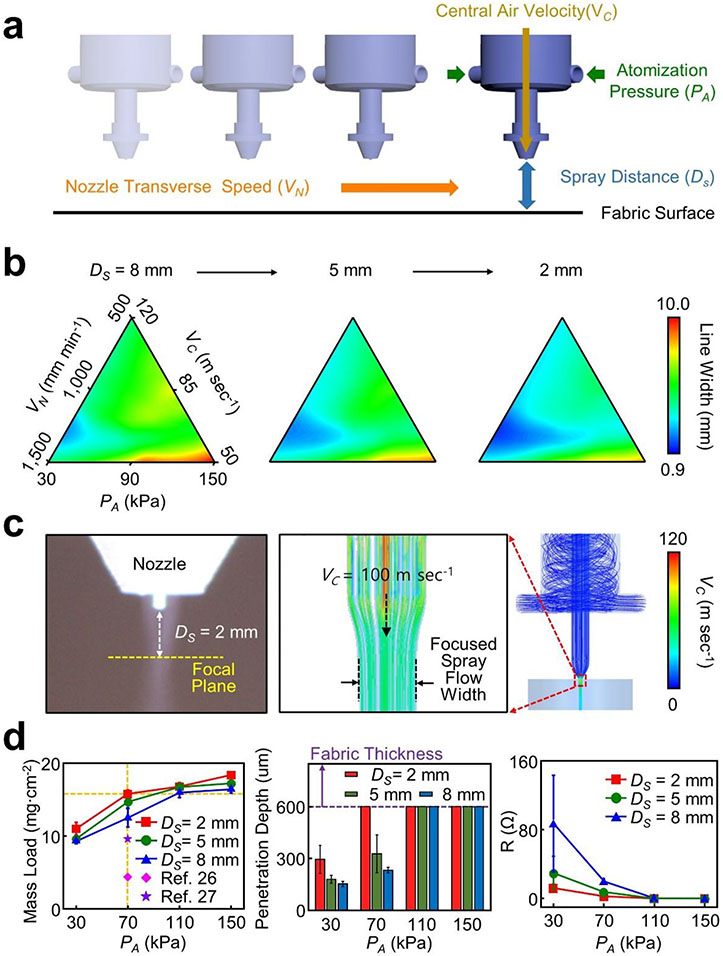
Identifying an optimal operational condition for the dual regime spray process is important to precisely define the line width (i.e., 0.9–10 mm), penetration depth (i.e., up to 600 μm-deep), and shape (i.e., straight or curvilinear lines) of conduction paths in fabrics. Figure 2a schematically illustrates the governing operational parameters of the dual regime spray process in terms of atomization gauge pressure (PA), nozzle transverse speed (VN), central air velocity (VC), and spray distance to fabrics (Ds). Figure 2b presents the effect of the governing operational parameters on spray resolution (i.e., line width) in a range of PA = 30–150 kPa, VN = 500–1,500 mm min−1, VC = 50–120 m sec−1, and DS = 2–8 mm. The spray resolution tended to increase with decreased PA or increased VC due to the narrow and deep interaction of the sprayed droplets with fabrics. Deep penetration throughout fabrics up to 600 μm in thickness occurred at PA = 70 kPa and VC = 100 m sec−1. The highest VN without compromising the highest spray resolution (i.e., 0.9 mm in line width) was 1,300 mm min−1 within a single working area (i.e., frame size of the CNC gantry) of 75 × 120 cm2 at a time. Excessive rise in PA or VN beyond a threshold (i.e., > 150 kPa or > 1,500 mm min−1) resulted in disruptive or non-uniform spray patterns due to the excessively or deficiently sprayed droplets, respectively (Figure S7a, Supporting Information). The spray resolution (i.e., minimum line width) increased up to 0.9 mm with increased VC up to 100 m sec−1 at fixed PA = 70 kPa and VN = 1,300 mm min−1 (Figure S7b, Supporting Information). Under this condition, the focal plane was formed at DS = 2 mm. Figure 2c summarizes the experimental and computational fluid dynamics (CFD) results of the spray flow. Details of the CFD domain and local grid are shown in Figure S8a, Supporting Information. The spray flow was narrowed by the central air flow (i.e., VC = 100 m sec−1) and then focused at the focal plan (i.e., DS = 2 mm), which yielded the highest spray resolution at 0.9 mm. Without the central air flow (i.e., VC = 0 m sec−1), the spray flow remained straight (Figure S8b, Supporting Information). A real-time video of the spray flow at VC = 0–100 m sec−1 is shown in Movie S2. Taken together, the highest spray resolution occurred at the optimal condition of PA = 70 kPa, VN = 1,300 mm min−1, VC = 100 m sec−1, and DS = 2 mm.
Figure 2. Optimal operational condition.
(a) Schematic illustration of the governing operational parameters in the dual regime spray process. (b) Effect of the governing operational parameters on spray resolution. (c) Representative experimental and CFD results of the spray flow. (d) Mass load, penetration depth, and electrical resistance of test units as a function of atomization pressures and spray distances.
Figure 2d presents the effect of PA and DS on mass load (left panel), penetration depth (middle panel), and electrical resistance (right panel) using test units (i.e., a conduction path in a size of 1.3 mm × 50 mm), all of which were sprayed in a Lycra fabric. The mass load increased as PA increased to reach the maximum (≈ 18 mg cm−2) at DS = 2 mm, which is substantially higher than those reported in previous studies.[24,25] The penetration depth also increased as PA increased where the deep penetration (> 600 μm) into fabrics occurred with PA ≥ 70 kPa at DS = 2 mm. The high mass load and deep penetration resulted in sufficiently low electrical resistance (< 2.6 Ω) at the optimal condition. The high mass load and deep penetration were mostly attributed to the kinetic energy gain of atomized droplets (i.e., ~2.7 μm in mass median average diameter) at high atomization pressure (i.e., PA ≥ 70 kPa).[26] Representative microscopy and SEM images of the test units are shown in Figure S9, Supporting Information. Representative examples of constructing e-textiles into a resistive heater and light-emitting diodes (LEDs) at the optimal condition are shown in Figure S10, Supporting Information.
2.3. Benchtop evaluations
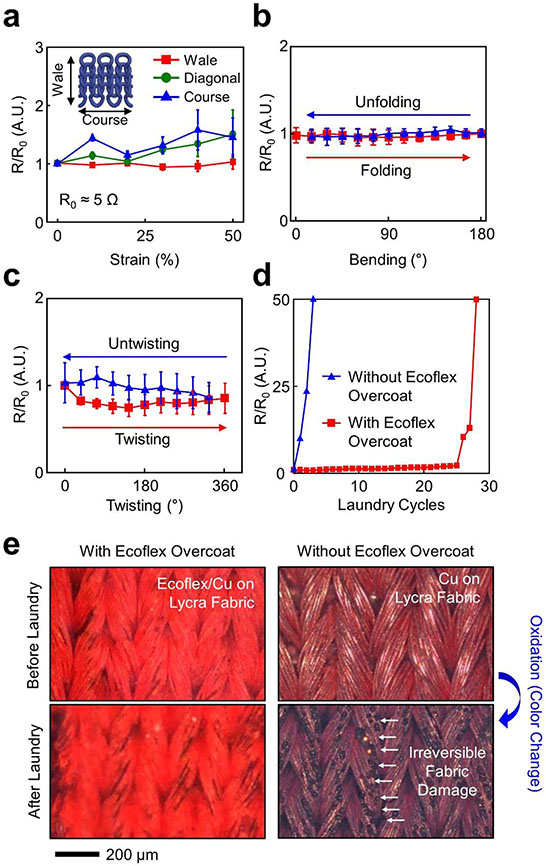
The implementation of e-textiles in ambulatory health monitoring requires their seamless contact to the skin without delamination against periodic strain cycles (i.e., on-body movements).[27] In addition, their long-term use in clinical practice also requires a sufficient level of mechanical flexibility, water vapor permeability, and comfortability.[28-30] Commercial stretch fabrics or garments already meet all these requirements as they are breathable, durable, and intrinsically stretchable to conform to a variety of body sizes and shapes with seamless fits. Therefore, there is a particular interest in converting commercial stretch fabrics or garments into multifunctional e-textiles. To demonstrate this, the dual regime spray process was applied to a Lycra fabric to generate test units (i.e., a conduction path in a size of 1.3 mm × 50 mm). The mechanical moduli of the test units were nearly identical as compared to the bare Lycra fabric (E ≈ 1.5 kPa), which increased up to 6.1 kPa with the presence of the Ecoflex overcoat (Figure S11, Supporting Information). Figure 3a presents the relative resistance change (R/R0) of the test units (n = 3 for each group) when stretched up to the intrinsic stretch limit (≈ 50%) of the Lycra fabric along three different directions: (1) wale (i.e., to the lengthwise column of the meandering loops), (2) diagonal, and (3) course (i.e., to the crosswise row of the meandering loops). No substantial change in the R/R0 appeared irrespective of the stretching direction. Irreversible fabric damages occurred when stretched beyond 50% mostly along diagonal and course directions (Figure S12a, Supporting Information), leading to an exponential increase of the R/R0 (Figure S12b, Supporting Information). Notably, the R/R0 barely changed when stretched even up to 150% along wale direction. This is likely attributed to the ultralow effective modulus (≈ 2.3 kPa) of the constituent “horseshoe”-like filamentary columns in the Lycra fabric that underwent large-scale, bending-dominant deformation when stretched along wale direction.[31] Figure S13 (Supporting Information) shows the experimental and finite element modelling (FEM) results of the single filamentary column, indicating that the maximum principal strain was 0%, 15.6%, and 38.5% at the elongation of 0%, 25%, and 50%, respectively. Consistently, the R/R0 barely changed against folding (Figure 3b) and twisting up to 360° (Figure 3c). No noticeable differences appeared in their deformation behaviors as compared to the bare Lycra fabric (Figure S14a, Supporting Information), exhibiting a negligible or insignificant change in the R/R0 following 1,000 cycles of the deformations (Figure S14b, Supporting Information).
Figure 3. Benchtop evaluations.
(a) R/R0 of test units under stretching up to 50%. (b) R/R0 of test units under folding and unfolding. (c) R/R0 of test units under twisting and untwisting up to 360°. (d) R/R0 of test units under a total of multiple laundry cycles with (red line) and without (blue line) the presence of the Ecoflex overcoat. (e) Microscope images of test units before and after the laundry cycles.
Figure 3d presents the impact of multiple laundry cycles on the R/R0 with (red line) and without (blue line) the presence of the Ecoflex overcoat using a standard washing machine (Whirlpool). Each laundry cycle involved the following steps: (1) 10-minute spinning in warm water at 30 °C with a fabric detergent (1 mg ml−1, Jacquard Synthrapol); (2) 5-minute rinsing in cold water, and (3) 2-minute spin-drying at room temperature. At least 3-hour sun-drying was followed for complete dry. The R/R0 barely changed against up to 25 laundry cycles with the presence of the Ecoflex overcoat and then sharply increased with more laundry cycles due to a partial loss of the Ecoflex overcoat (Figure S15, Supporting Information). Without the presence of the Ecoflex overcoat, the R/R0 increased immediately after each laundry cycle due to irreversible fabric damages or oxidations (i.e., color change) (Figure 3e). Importantly, the water vapor permeability of the e-textiles remained similar to that of the bare Lycra fabric when the areal fill factor of the Ecoflex overcoat remained < 25% (Figure S16, Supporting Information).
2.4. Large-scale application in horses
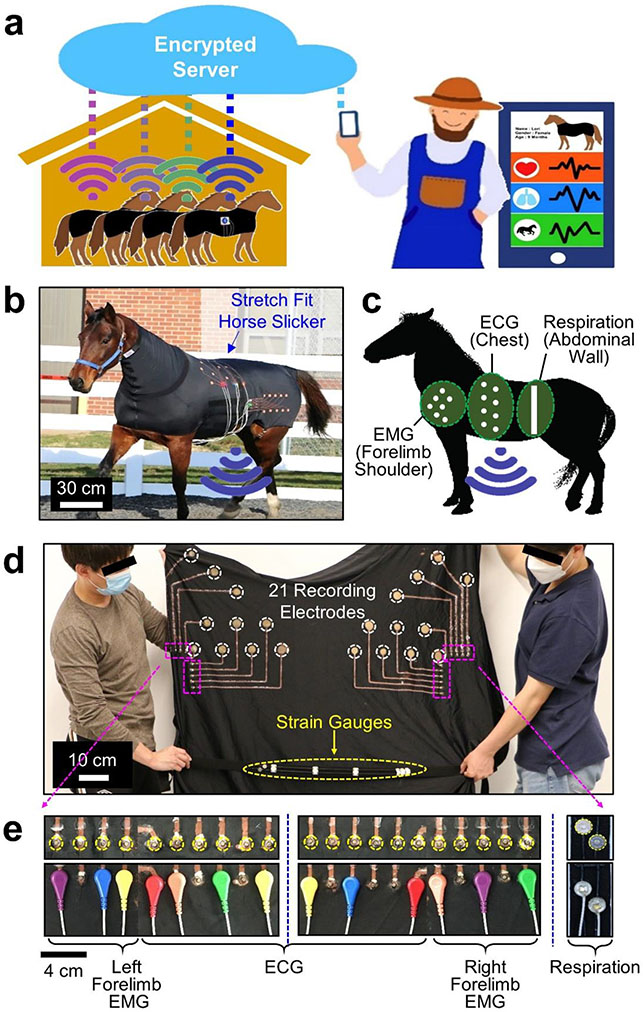
Increasing demand for remote health monitoring of farm or household animals calls for an effective wearable sensing platform to continuously collect vital health signals under ambulatory conditions.[32-34] Despite the great promise of e-textiles in this context, a rapid prototyping of custom sensor designs remains limited particularly at large scale for large animals. To address this unmet need, a pilot study was carried out using the dual regime spray process to produce multimodal e-textiles for a large animal such as a horse. Tailored sensor designs were incorporated into commercial stretch horse blankets to support the continuous monitoring of heart activity, muscle movement, and respiration pattern in horses under ambulatory conditions. Figure 4a schematically depicts the envisioned concept of the remote health monitoring of horses, supported by wireless (i.e., Bluetooth) communications with a caregiver via an encrypted server. In this pilot study, Standardbred female horses (n = 2; 11–16 years old; 417–449 kg) were included in accordance with the Purdue Institutional Animal Care and Use Committee (approved protocol number: 1111000176). The horses were considered healthy with a median body condition score of > 5 out of 9 points. Prior to this study, the horses were trained to perform standardized exercises such as walking, trotting, and galloping during lunging in a round pen. The horses were housed in a stall, fed hay, and bedded on shavings.
Figure 4. Large-scale application in horses.
(a) Schematic illustration of the envisioned concept for the ambulatory health monitoring of horses. (b) Photograph of a horse in a custom e-textile. (c) Measurement locations of ECG, EMG, and abdominal strains in a horse. (d) Photograph of the e-textile. (e) Photographs of the snap button ends in the e-textile.
Figure 4b provides a photograph of a horse in a custom e-textile built upon a commercial stretch horse blanket (Lycra fabric; Schneiders). The e-textile was configured into a tailored sensor design for the simultaneous monitoring of electrocardiogram (ECG) signals (i.e., heart activity), electromyogram (EMG) signals (i.e., muscle movement), and abdominal strains (i.e., respiration pattern) from the chest, forelimb shoulder, and abdominal wall of the horse, respectively (Figure 4c). A total of 21 recording electrodes and 2 strain gauges were rationally distributed across the entire area (100 cm × 130 cm) of the stretch horse blanket to meet specific geometric demands (Figure 4d). A total of 21 snap button ends (Florida Research Instruments) were glued using a waterproof textile adhesive (Liquid Stitch) on the peripheral edge of the stretch horse blanket in a minimally obtrusive manner (Figure 4e). The snap button ends were used for wire connections to a custom-built portable data acquisition unit (74 cm × 58 mm × 32 mm; 150 g) clipped on the stretch horse blanket. Several of the snap button ends were appropriately chosen to define desired measurement locations by considering horse-by-horse variations in body size and shape. The portable data acquisition unit was modified from a commercial unit (BioRadio; GLNeuroTech), allowing for (1) multichannel data acquisition (up to 8 single-ended channels) at a sample rate of 250–16,000 Hz; (2) battery powering with the capacity of 420 mAh at 3.7 V; and (3) wireless (i.e., Bluetooth) data communications at 190 kbps within the maximum transmission range of 30 meters. Details of the portable data acquisition unit are shown in Figure S17, Supporting Information. Several different designs of the e-textiles were produced in various types of stretch horse blankets, including full zip blanket hood (Lycra), faceless pull-on full body blanket (Lycra), and mesh full zip blanket hood (polyester), without noticeable difference in overall quality (Figure S18, Supporting Information). For all these cases, the areal fill factor of the Ecoflex overcoat was kept at < 25% in order to maintain the water vapor permeability of the e-textiles as similar as that of their bare horse blankets. As a result, no sign of discomfort was observed in the horses while freely walking, trotting, and galloping in a round pen or resting and eating in a stall (Figure S19, Supporting Information).
2.5. Pilot field tests in ambulatory health monitoring
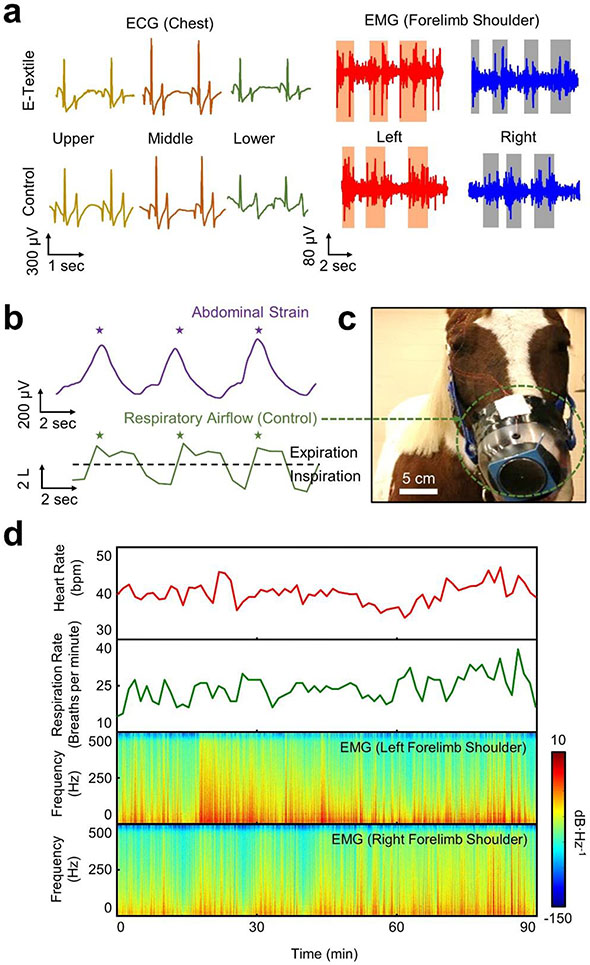
Figure 5a shows the representative measurement results of ECG (left panel) and EMG (right panel) signals, each of which was obtained from the upper, middle, and lower chest and the left and right forelimb shoulders of a horse under ambulatory conditions. Both the ECG and EMG signals were captured on the skin, without shaving haircoat, at a sampling rate of 1 kHz per channel with 24-bit resolution. A reference (ground) electrode was placed on tarsal bone. For the measurements, an electrolyte gel (Parker) was applied to the recording electrodes for enhanced electrical contact to the skin. The ECG signals exhibited no delay between P and QRS-waves. The recording electrodes in the e-textiles exhibited the electrical impedance of 121–442 Ω in a bandwidth of 20–500 Hz which is within a typical range of commercial recording electrodes (250–374 Ω) (Figure S20a, Supporting Information). For control measurements, commercial equine self-adhesive electrodes (H124SG; Covidien) were attached to the skin after shaving haircoat to reduce noise level (Figure S20b, Supporting Information). Notably, the e-textiles formed a seamless, tight interface with the skin under ambulatory conditions, even without the use of adhesives, thereby enabling the high-fidelity recording of ECG and EMG signals. In turn, the signal quality in terms of the amplitude, duration, and relative onset and offset times of the ECG and EMG signals was comparable between the measurements using the e-textiles and the control recording electrodes without and with shaving haircoat, respectively. Occasional motion artifacts were observed in the ECG signals during the abrupt change of body postures, which were alleviated by applying a sufficient amount (≥ 2 g) of the electrolyte gel.[35,36]
Figure 5. Pilot field tests in ambulatory health monitoring.
(a) Representative measurement results of ECG (left panel) and EMG (right panel) signals in a horse while walking. (b) Representative measurement results of abdominal strains (top panel) as compared to control measurements using a conventional pneumotachograph (bottom panel). (c) Photograph of the pneumotachograph inserted into the muzzle of a horse. (d) Time-series and spectral dataset of heart rate, respiration rate, and forelimb movement in a horse while eating, wandering, and resting for 90 minutes. The colors indicate the amplitude spectral density.
Figure 5b shows the representative measurement results of abdominal strains (i.e., respiration pattern) obtained using the strain gauges within the e-textiles (top panel) as compared to control measurements (bottom panel) using a conventional pneumotachograph (#4 Fleisch; EMKA Technologies). Details of the strain gauges are shown in Figure S21 (Supporting Information). The gauge factor of the strain gauges was measured as ~5.2 at the applied strain ranging from 0 to 15%. Figure 5c shows the enlarged image of the pneumotachograph that was inserted in a facemask placed on the muzzle of the horse. The pneumotachograph was wired to an external differential pressure transducer (DP-/45-14; Validyne Engineering) while the horse was tethered still during the measurements. The tethered measurements inevitably posed discomfort and disruption in continuous recording under ambulatory conditions, as also reported in previous studies using the pneumotachograph.[37,38] Both the strain and respiratory airflow waveforms were clearly distinguishable with respect to inspiratory and expiratory abdominal movement, exhibiting the respiration rate of 12 breaths per minute under the condition. Figure 5d presents the time-series and spectral dataset of heart rate, respiration rate, and forelimb movement in the horse while resting in a stall for 90 minutes. The average heart rate and respiration rate were measured as 40 bpm and 24 breaths per minute, respectively. The forelimb movement was also clearly distinguishable without showing substantial motion artifacts through bandpass filtering at the cut-off frequency of 20–500 Hz.
A particular utility of the e-textiles may also exist in the routine ambulatory monitoring of cardiac and muscle activity in horse athletes to better understand exercise physiology in daily practical training.[39,40] To demonstrate this, Figure S22 (Supporting Information) summarizes the quantitative details of ambulatory ECG and EMG signals obtained from the chest and forelimb shoulders of a horse while trotting, walking, and resting in an arena for 15 minutes in a random order. The high-fidelity recording of the signals was maintained throughout the entire testing period without any sign of discomfort or skin irritation in the horse (Movie S3). All the signals were simultaneously collected through multichannel data acquisitions at a sampling frequency of 1 kHz per channel from a distance via Bluetooth communications. The power consumption for the wireless monitoring was approximately 50 mA with a battery life of up to 8 hours on a single charge.
3. Conclusion
The results presented herein represent a unique approach in converting arbitrary fabrics into custom-designed e-textiles at high spatial resolution across a large area without significantly compromising the intrinsic fabric properties. This approach involves using a programmable dual regime spray that enables the direct writing of custom sensor designs at substantially higher resolution (0.9 mm in line width) and conductivity (≥ 9,400 S cm−1) compared to other approaches using common sprays. The resulting e-textiles fit tightly to the body in various sizes and shapes while anchoring the recording electrodes in place under ambulatory conditions. Leveraging these features, the e-textiles provide superior measurement accuracy and fidelity in capturing physiological and electrophysiological signals over current measurement methods. Pilot field tests in a large animal, such as a horse, demonstrate the scalability and utility of the e-textiles in remote health monitoring. The established platform technology can be potentially tailored for humans in health and diseases.
Supplementary Material
Acknowledgements
T.C. and S.A. contributed equally to this work. C.H.L. acknowledges funding supports from the National Institute of Health (NIH) National Institute of Biomedical Imaging and Bioengineering (NIBIB) (1R21EB026099-01A1) and the National Science Foundation (NSF) Civil, Mechanical and Manufacturing Innovation (CMMI) (1928784) for the initial exploratory studies. C.H.L. also acknowledges funding supports from the Leslie A. Geddes Endowment and the SMART film at Purdue University. S.A. acknowledges a scholarship support through the Office of Overseas Scholarship Program from the Republic of Turkey Ministry of National Education.
Footnotes
Experimental Section
A detailed description of procedures and characterization methods are available in the Supporting Information.
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Conflict of Interest
The authors declare no conflict of interest.
Contributor Information
Taehoo Chang, School of Materials Engineering, Purdue University, West Lafayette, IN 47907, USA.
Semih Akin, School of Mechanical Engineering, Purdue University, West Lafayette, IN 47907, USA.
Min Ku Kim, Weldon School of Biomedical Engineering, Purdue University, West Lafayette, IN 47907, USA.
Laura Murray, Department of Veterinary Clinical Sciences, Purdue University, West Lafayette, IN 47907, USA.
Bongjoong Kim, School of Mechanical Engineering, Purdue University, West Lafayette, IN 47907, USA.
Seungse Cho, Weldon School of Biomedical Engineering, Purdue University, West Lafayette, IN 47907, USA.
Sena Hur, Weldon School of Biomedical Engineering, Purdue University, West Lafayette, IN 47907, USA.
Sengul Teke, School of Mechanical Engineering, Purdue University, West Lafayette, IN 47907, USA; Secant Group, LLC, Telford, PA 18969, USA.
Laurent Couetil, Department of Veterinary Clinical Sciences, Purdue University, West Lafayette, IN 47907, USA.
Martin Byung-Guk Jun, School of Mechanical Engineering, Purdue University, West Lafayette, IN 47907, USA; Indiana Manufacturing Competitiveness Center, Purdue University, West Lafayette, IN 47907, USA.
Chi Hwan Lee, School of Materials Engineering, Purdue University, West Lafayette, IN 47907, USA; School of Mechanical Engineering, Purdue University, West Lafayette, IN 47907, USA; Weldon School of Biomedical Engineering, Purdue University, West Lafayette, IN 47907, USA; Birck Nanotechnology Center, Purdue University, West Lafayette, IN 47907, USA.
References
- [1].Wang L, Fu X, He J, Shi X, Chen T, Chen P, Wang B, Peng H, Adv. Mater 2020, 32, 1901971. [DOI] [PubMed] [Google Scholar]
- [2].Shi J, Liu S, Zhang L, Yang B, Shu L, Yang Y, Ren M, Wang Y, Chen J, Chen W, Chai Y, Tao X, Adv. Mater 2020, 32, 1901958. [DOI] [PubMed] [Google Scholar]
- [3].Yan W, Page A, Nguyen-Dang T, Qu Y, Sordo F, Wei L, Sorin F, Adv. Mater 2019, 31, 1802348. [DOI] [PubMed] [Google Scholar]
- [4].Tian X, Lee PM, Tan YJ, Wu TLY, Yao H, Zhang M, Li Z, Ng KA, Tee BCK, Ho JS, Nat. Electron 2019, 2, 243. [Google Scholar]
- [5].La T-G, Qiu S, Scott DK, Bakhtiari R, Kuziek JWP, Mathewson KE, Rieger J, Chung H-J, Adv. Healthc. Mater 2018, 7, 1801033. [DOI] [PubMed] [Google Scholar]
- [6].Yin L, Kim KN, Lv J, Tehrani F, Lin M, Lin Z, Moon J-M, Ma J, Yu J, Xu S, Wang J, Nat. Commun 2021, 12, 1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jayathilaka WADM, Qi K, Qin Y, Chinnappan A, Serrano-García W, Baskar C, Wang H, He J, Cui S, Thomas SW, Ramakrishna S, Adv. Mater 2019, 31, 1805921. [DOI] [PubMed] [Google Scholar]
- [8].Dong K, Peng X, Wang ZL, Adv. Mater 2020, 32, 1902549. [DOI] [PubMed] [Google Scholar]
- [9].Cao R, Pu X, Du X, Yang W, Wang J, Guo H, Zhao S, Yuan Z, Zhang C, Li C, Wang ZL, ACS Nano 2018, 12, 5190. [DOI] [PubMed] [Google Scholar]
- [10].Guo R, Yu Y, Xie Z, Liu X, Zhou X, Gao Y, Liu Z, Zhou F, Yang Y, Zheng Z, Adv. Mater 2013, 25, 3343. [DOI] [PubMed] [Google Scholar]
- [11].Hu L, Cui Y, Energy Environ. Sci 2012, 5, 6423. [Google Scholar]
- [12].Uzun S, Schelling M, Hantanasirisakul K, Mathis TS, Askeland R, Dion G, Gogotsi Y, Small 2021, 17, 2006376. [Google Scholar]
- [13].Shin YE, Lee JE, Park Y, Hwang SH, Chae HG, Ko H, J. Mater. Chem. A 2018, 6, 22879. [Google Scholar]
- [14].Carey T, Cacovich S, Divitini G, Ren J, Mansouri A, Kim JM, Wang C, Ducati C, Sordan R, Torrisi F, Nat. Commun 2017, 8, 1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jin H, Matsuhisa N, Lee S, Abbas M, Yokota T, Someya T, Adv. Mater 2017, 29, 1605848. [DOI] [PubMed] [Google Scholar]
- [16].Matsuhisa N, Kaltenbrunner M, Yokota T, Jinno H, Kuribara K, Sekitani T, Someya T, Nat. Commun 2015, 6, 7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Akin S, Gabor T, Jo S, Joe H, Tsai J-T, Park Y, Lee CH, Park MS, Jun MB-G, J. Micro Nano-Manufacturing 2020, 8, 024511. [Google Scholar]
- [18].Yang Y, Wei X, Zhang N, Zheng J, Chen X, Wen Q, Luo X, Lee C-Y, Liu X, Zhang X, Chen J, Tao C, Zhang W, Fan X, Nat. Commun 2021, 12, 1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yoon J, Jeong Y, Kim H, Yoo S, Jung HS, Kim Y, Hwang Y, Hyun Y, Hong W-K, Lee BH, Choa S-H, Ko HC, Nat. Commun 2016, 7, 11477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Park H, Kim JW, Hong SY, Lee G, Lee H, Song C, Keum K, Jeong YR, Jin SW, Kim DS, Ha JS, ACS Nano 2019, 13, 10469. [DOI] [PubMed] [Google Scholar]
- [21].Jinno H, Fukuda K, Xu X, Park S, Suzuki Y, Koizumi M, Yokota T, Osaka I, Takimiya K, Someya T, Nat. Energy 2017, 2, 780. [Google Scholar]
- [22].Choi S, Han SI, Jung D, Hwang HJ, Lim C, Bae S, Park OK, Tschabrunn CM, Lee M, Bae SY, Yu JW, Ryu JH, Lee S-W, Park K, Kang PM, Lee WB, Nezafat R, Hyeon T, Kim D-H, Nat. Nanotechnol 2018, 13, 1048. [DOI] [PubMed] [Google Scholar]
- [23].Svanidze E, Besara T, Fevsi Ozaydin M, Tiwary CS, Wang JK, Radhakrishnan S, Mani S, Xin Y, Han K, Liang H, Siegrist T, Ajayan PM, Morosan E, Sci. Adv 2016, 2, e1600319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Root W, Aguiló-Aguayo N, Pham T, Bechtold T, Surf. Coatings Technol 2018, 348, 13. [Google Scholar]
- [25].Landsiedel J, Root W, Schramm C, Menzel A, Witzleben S, Bechtold T, Pham T, Nano Research 2020, 13, 10. [Google Scholar]
- [26].Laserna JJ, Moros J, Álvarez-Trujillo LA, Lazic V, Appl. Opt 2017, 56, 13. [DOI] [PubMed] [Google Scholar]
- [27].Jin H, Nayeem MOG, Lee S, Matsuhisa N, Inoue D, Yokota T, Hashizume D, Someya T, ACS Nano 2019, 13, 7905. [DOI] [PubMed] [Google Scholar]
- [28].Jang K-I, Han SY, Xu S, Mathewson KE, Zhang Y, Jeong J-W, Kim G-T, Webb RC, Lee JW, Dawidczyk TJ, Kim RH, Song YM, Yeo W-H, Kim S, Cheng H, Il Rhee S, Chung J, Kim B, Chung HU, Lee D, Yang Y, Cho M, Gaspar JG, Carbonari R, Fabiani M, Gratton G, Huang Y, Rogers JA, Nat. Commun 2014, 5, 4779. [DOI] [PubMed] [Google Scholar]
- [29].Uzun S, Seyedin S, Stoltzfus AL, Levitt AS, Alhabeb M, Anayee M, Strobel CJ, Razal JM, Dion G, Gogotsi Y, Adv. Funct. Mater 2019, 29, 1905015. [Google Scholar]
- [30].Yetisen AK, Qu H, Manbachi A, Butt H, Dokmeci MR, Hinestroza JP, Skorobogatiy M, Khademhosseini A, Yun SH, ACS Nano 2016, 10, 3042. [DOI] [PubMed] [Google Scholar]
- [31].Jang K-I, Chung HU, Xu S, Lee CH, Luan H, Jeong J, Cheng H, Kim G-T, Han SY, Lee JW, Kim J, Cho M, Miao F, Yang Y, Jung HN, Flavin M, Liu H, Kong GW, Yu KJ, Il Rhee S, Chung J, Kim B, Kwak JW, Yun MH, Kim JY, Song YM, Paik U, Zhang Y, Huang Y, Rogers JA, Nat. Commun 2015, 6, 6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Dixon TA, Williams TC, Pretorius IS, Nat. Commun 2021, 12, 388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhang M, Wang X, Feng H, Huang Q, Xiao X, Zhang X, J. Clean. Prod 2021, 312, 127712. [Google Scholar]
- [34].Schmidt-Traub G, Obersteiner M, Mosnier A, Nat. 2019, 569, 181. [DOI] [PubMed] [Google Scholar]
- [35].Kim MK, Kantarcigil C, Kim B, Baruah RK, Maity S, Park Y, Kim K, Lee S, Malandraki JB, Avlani S, Smith A, Sen S, Alam MA, Malandraki G, Lee CH, Sci. Adv 2019, 5, eaay3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Khan Y, Garg M, Gui Q, Schadt M, Gaikwad A, Han D, Yamamoto NAD, Hart P, Welte R, Wilson W, Czarnecki S, Poliks M, Jin Z, Ghose K, Egitto F, Turner J, Arias AC, Adv. Funct. Mater 2016, 26, 8764. [Google Scholar]
- [37].Arroyo MG, Couëtil LL, Nogradi N, Kamarudin MM, Ivester KM, J. Vet. Intern. Med 2016, 30, 1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hoffman A, Kuehn H, Riedelberger K, Kupcinskas R, Miskovic MB, J. Appl. Physiol 2001, 91, 2767. [DOI] [PubMed] [Google Scholar]
- [39].Munsters CCBM, Kingma BRM, van den Broek J, Sloet van Oldruitenborgh-Oosterbaan MM, Prev. Vet. Med 2020, 179, 105010. [DOI] [PubMed] [Google Scholar]
- [40].Guidi A, Lanata A, Valenza G, Scilingo EP, Baragli P, J. Vet. Behav 2017, 17, 19. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.