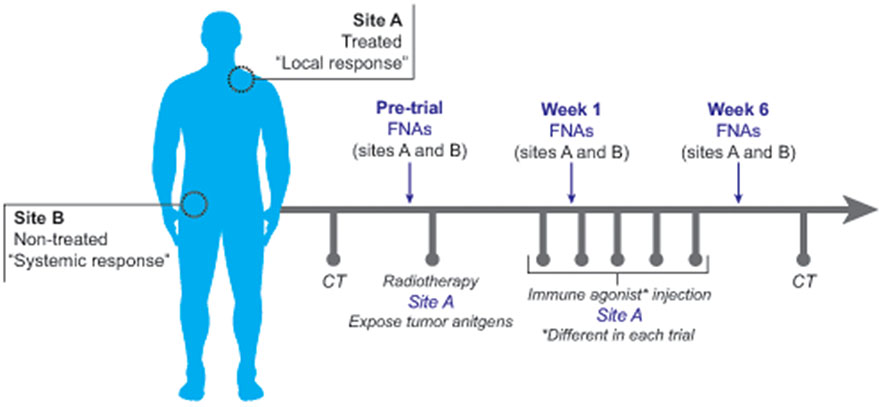
Figure 1.
Overview of FNA methodology in clinical trials. Two FNA sites were identified in each patient including “Site A”, the treated site, and “Site B”, the non-treated site. Following pre-treatment CT scans, an initial pre-treatment FNA was performed at sites A and B. Low-dose radiation therapy followed by the first immunotherapy dose was then administered to Site A only. Eight days later, a second FNA was performed at sites A and B (Week 2). A third FNA was performed at sites A and B at week 6, subsequent to the final immunotherapy dose. CT scans were performed at intervals defined by each trial protocol. Each trial utilized different immune agonists or combinations of immune agonists.