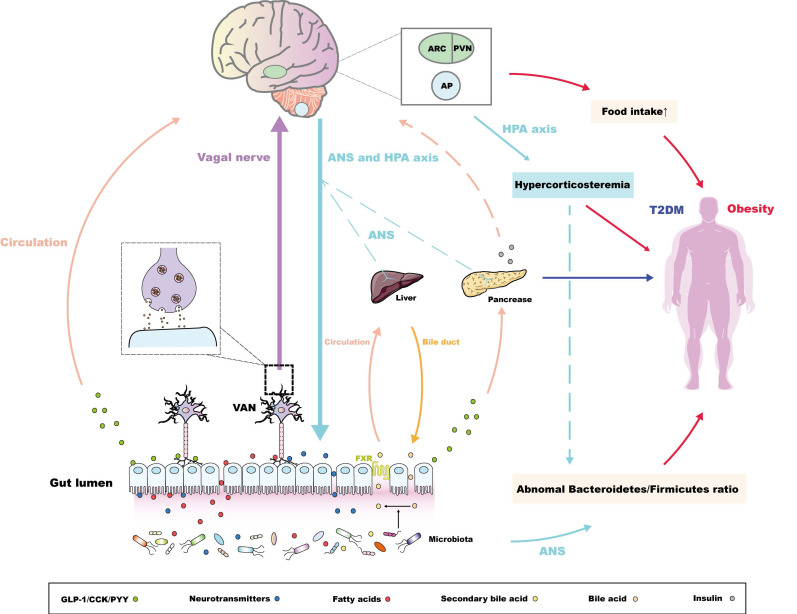
Figure 1.
Bidirectional pathways within the MGBA concerning the pathogenesis of metabolic diseases. For the pathway from the gut to the brain, the intestinal microbiota secretes metabolites to promote the release of intestinal peptide hormones and neurotransmitters 37,38,47, along with the conversion of bile acids. The conjugated bile acids are transferred from bile duct and combine the farnesoid X receptor (FXR) in ileal enterocytes to be reabsorbed into the liver via circulation 52. These endocrine signals induced by microbiota could directly or indirectly maintain the peripheral metabolic homeostasis. Alternatively, intestinal microbiota-derived endocrine signals are associated with metabolic-related encephalic regions through the "enteroendocrine cells-enteric nervous system-vagus nerve" pathway 55,56. The molecular pathways via the arcuate nucleus (ARC), the paraventricular nucleus (PVN), and the area postrema (AP) mainly control the balance of appetite, regulating the occurrence and development of obesity 27,35,36,57,58, while these pathways could also regulate glucose uptake of the pancreas, liver through the autonomic nerve system (ANS) 64,65. Notably, the central insulin resistance, which has also been proven to be pathogeny of the T2DM 66,67, is also induced by the abnormal sensitivity or secretion of peripheral insulin initiated by intestinal microbiota or its metabolites 69. For the pathway from the brain to the gut, the anomalous ANS or HPA axis-derived signals could regulate the Bacteroidetes/Firmicutes ratio, which is vital in the development of obesity 77,78. Interestingly, abnormal HPA axis signatures also contribute to hypercorticosteremia, which constitutes risk factors of obesity 78. Following studies could further investigate the relationship between hypercorticosteremia and the 'brain-microbiota-obesity' axis.