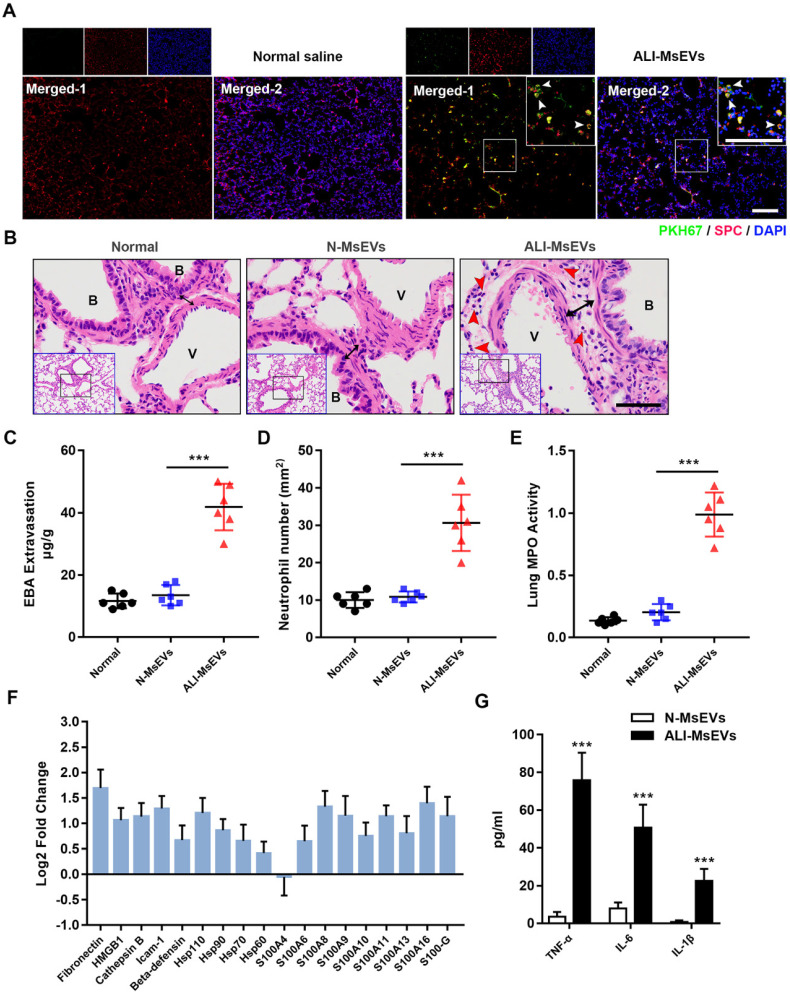
Figure 3.
MsEVs derived from alveolar macrophages in the early stage of ALI encapsulate DAMPs and mediate inflammation. (A) After tracheal drip of PKH67-labeled PyrBDs for 4 h, immunofluorescent staining showed that PKH67 co-localized with SPC, indicating the uptake of PyrBDs by lung epithelial cells. The white arrow points to undegraded PyrBDs (green); bar = 100 μm. The number of EVs is one million per mouse. SPC (red); DAPI (blue). (B) H&E-stained cross-section of the lung from N-MsEVs (MsEVs from the normal group) and ALI-MsEVs (MsEVs from the ALI-1h group) exposed mice at 4 h shows interstitial edema (indicated by black arrows). The red arrow points to neutrophils. Scale bars: 50 μm. B, trachea; V, blood vessel. Images are representative of six animals. (C) Assessment of the degree of lung vascular leakage (EBA extravasation). (D) Quantitative analysis of neutrophil infiltration in the lungs. (E) Quantitative analysis of lung tissue MPO activity. (F) PRM for whole-protein analysis of MsEVs, from which DAMP-related molecular proteins were screened. EVs pellets were isolated from BALF collected from control mouse or 1 h post-LPS. The results are expressed as log2 fold change (n = 5). (G) TNF-α, IL-6, and IL-1β content of MsEVs. The EVs pellets were ultrasonically broken, the supernatant was obtained by centrifugation at 14,000g, and cytokine content was detected by ELISA kit. Compared with N-MsEVs, ALI-MsEVs contain high levels of TNF-α, IL-6, and IL-1β. Data are expressed as the mean ± SD, n = 6. ***P < 0.001.