Figure 2.
Assessing SARS-2 RBD immune focusing via serum analysis from cohorts
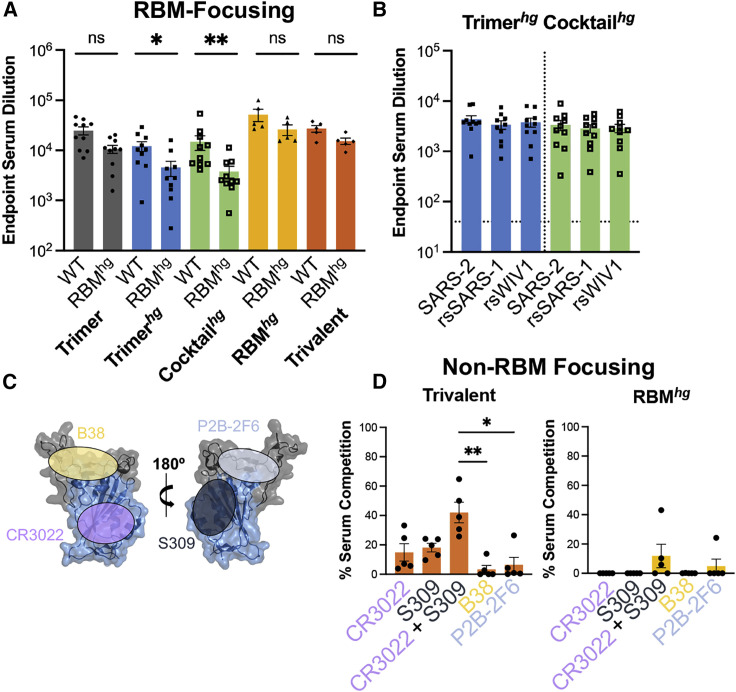
(A) Serum following immunizations was assayed by ELISA on day 35 with the wild-type SARS-2 RBD and RBMhg. Statistical significance was determined using a Mann-Whitney U test (∗p < 0.05, ∗∗p < 0.01). Bars represent mean ± SE.
(B) Day 35 serum samples from the Trimerhg and Cocktailhg cohorts showed significantly less binding to the SARS-1 and WIV1 RBDs compared with the SARS-2 RBD (Figures S3B and S3C). However, when assayed against rsSARS-1 and rsWIV1 RBDs, these sera no longer show statistically significant differences in binding compared with the SARS-2 RBD, as determined using a Kruskal-Wallis test with post hoc analysis using Dunn’s test corrected for multiple comparisons. Bars represent mean ± SE.
(C) Approximate locations of representative Ab epitopes from each of the four SARS-2 RBD-directed Ab classes (Barnes et al., 2020; PDB: 6M0J).
(D) Percent competition in ELISAs using day 35 mouse sera in the presence of competing IgGs versus a no-IgG control. SARS-2 RBD was the coating antigen. Statistical significance was determined using a Kruskal-Wallis test with post hoc analysis using Dunn’s test corrected for multiple comparisons, and pairwise comparisons pictured without bars were not significant (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001); ns, not significant.
See also Figure S3.