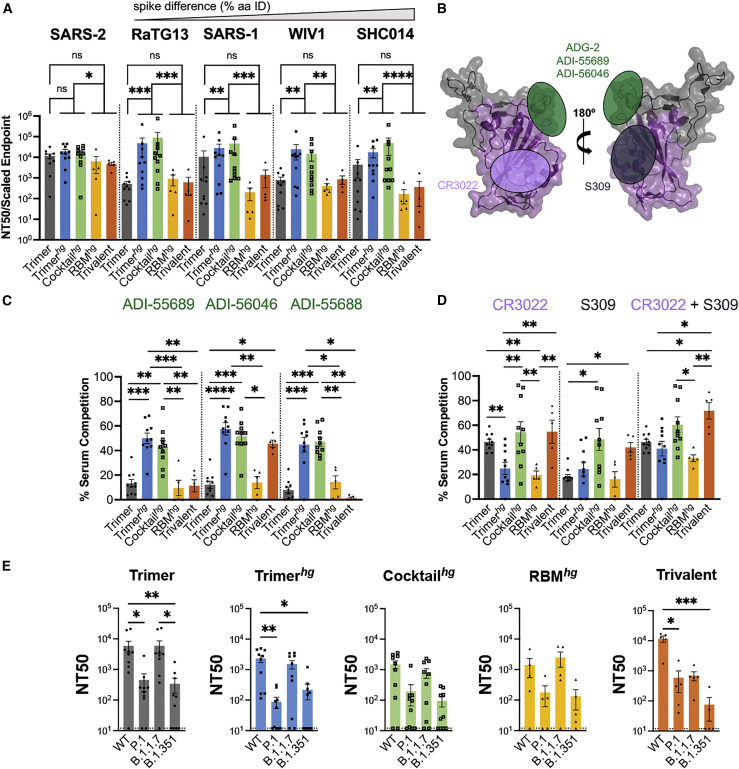
Figure 3.
Potency and characterization of the SARS-like coronavirus neutralization response
(A) Day 35 serum from all mice was assayed for neutralization against SARS-2, RaTG13, SARS-1, WIV1, and SHC014 pseudoviruses (arranged in order of genetic similarity of the full-length spike to SARS-2). Neutralization potency was computed using scaled endpoint serum ELISA titers. Statistical significance was determined using a Kruskal-Wallis test with post hoc analysis using Dunn’s test corrected for multiple comparisons (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001). Bars represent mean ± SE.
(B) Approximate locations of representative Ab epitopes from the two non-RBM-directed SARS-2 RBD-directed Ab classes (Barnes et al., 2020) and ADG-2-like Abs on the WIV1 RBD (PDB: 6M0J).
(C and D) Ab competition ELISAs with WIV1 RBD as the coating antigen. Bars show the mean percent binding lost, with error bars representing the standard error of the mean. Comparisons were performed using a Kruskal-Wallis test with post hoc analysis using Dunn’s test corrected for multiple comparisons, and pairwise comparisons pictured without bars were not significant (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001). Bars represent mean ± SE.
(E) Day 35 serum was assayed against SARS-2 variant pseudoviruses for neutralization. Statistical significance was determined using a Kruskal-Wallis test with post hoc analysis using Dunn’s test corrected for multiple comparisons, and pairwise comparisons pictured without bars were not significant (∗p < 0.05, ∗∗p < 0.01). Bars represent mean ± SE.
See also Figure S4).