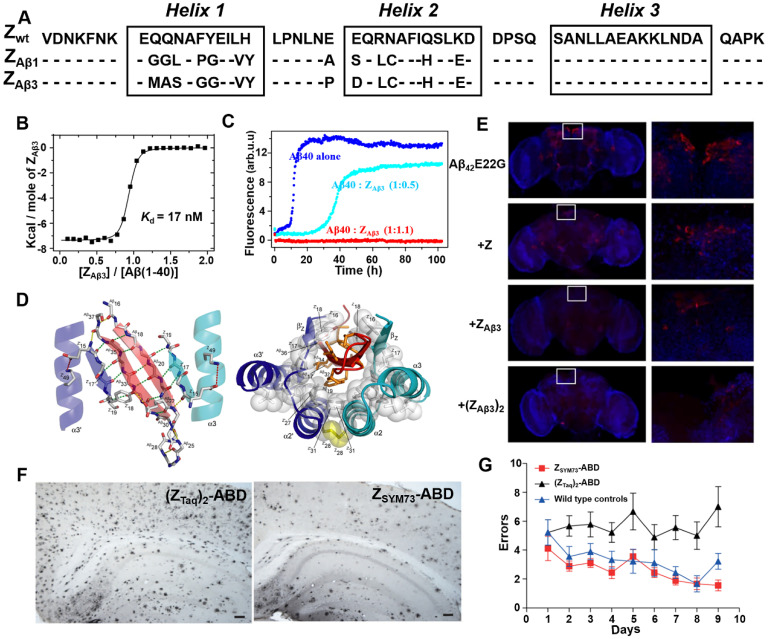
Figure 5.
Phage display-derived affibody targeting Aβ and their functional characterization. (A) Amino acid sequences of ZAβ1 and ZAβ3 selected against the Aβ peptide. (B) Titration of ZAβ3 dimer into Aβ (1-40) monitored by ITC. (C) Aggregation time course of Aβ (1-40) in the absence (blue) and presence of 0.5 (cyan) or 1.1 (red) molar equivalents of ZAβ3 dimer monitored by ThT fluorescence. (D) Structure of the ZAβ3: Aβ (1-40) complex. (E) Immunofluorescence analysis of intact brains using Aβ-binding antibodies 6E10/4G8 from flies expressing Aβ42E22G alone or in combination with Z domain control, ZAβ3 or (ZAβ3)2 affibody constructs. Anti-Aβ immunostaining is shown in red, with a nuclear counterstain (TOTO-3) shown in blue. (F) Immunohistochemical images of total amyloid burden using Aβ-binding antibodies 6E10/4G8 on brain sections from ZSYM73-ABD or (ZTaq)2-ABD control-treated APP/PS1 Tg mice. ZSYM73-ABD reduced total amounts of amyloid burden in the regions of cortex and hippocampus. (G) Radial arm maze testing of the ZSYM73-ABD (red) and (ZTaq)2-ABD (black) treated AD Tg mice. The ZSYM73-ABD treated mice navigated the maze with fewer errors than (ZTaq)2-ABD group. (B-D) Reprinted with permission from 49. Copyright (2008) National Academy of Sciences, U.S.A. (E) Licensed under a Creative Commons Attribution (CC BY) license. (F, G) Licensed under a Creative Commons Attribution (CC BY) license.