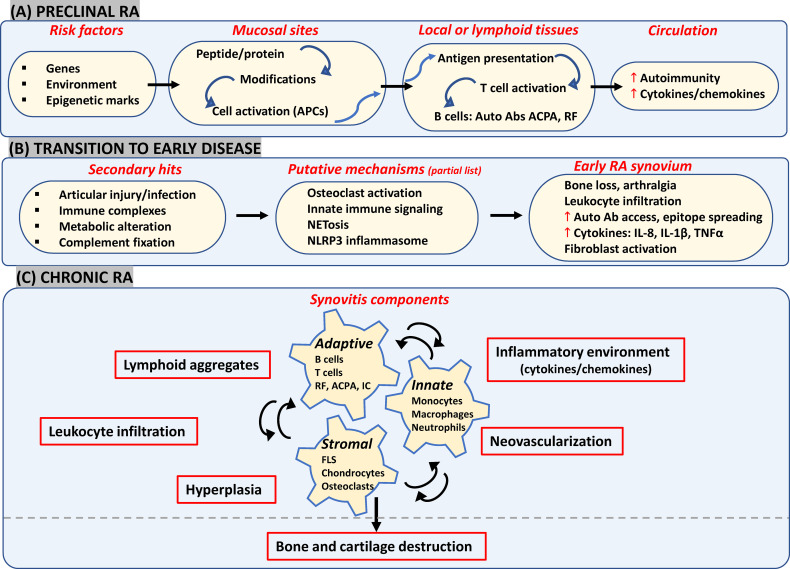
Figure 1.
Likely components in emerging view of RA onset and progression. (A) Interactions between genetic and environmental factors may trigger post-translational modifications in proteins (such as citrullination and carbamylation) at mucosal surfaces (such as gut, lungs and joints). In a genetically susceptible individual, this can activate innate immunity leading eventually to presentation of modified proteins by APCs like dendritic cells to T-cells in secondary lymphoid tissues. Subsequent systemic autoimmunity is characteristic of preclinical asymptomatic RA. (B) Multiple secondary hits may trigger mechanisms leading to synovial inflammation. Local osteoclast activation by ACPAs may cause early arthralgia and leukocyte recruitment. Local tissue insults, immune complex (IC) formation and complement activation may trigger synovial innate cells to increase cytokine production and vascular leakage. Release of NETosis and activation of NLRP3 inflammasome in macrophages could be other triggering mechanisms. (C) FLS get activated via inflammatory mediators released by lymphocytes, neutrophils and myeloid cells which further enhance inflammatory loops. Resulting synovitis works towards bone and joint damage via osteoclasts and FLS in intimal lining along with changes in tissue architecture characterized by ectopic lymphoid structures responsible for epitope spreading and angiogenesis to support cellular heterogeneity and effector functions in hypoxic microenvironment. APCs, antigen presenting cells; ACPA, anti-citrullinated protein antibodies; IC, immune complex; NETosis, neutrophil extracellular traps; NLRP3, nucleotide-binding, oligomerization domain (NOD)-like receptor family, pyrin domain containing 3; FLS, fibroblast-like synoviocytes; RF, rheumatoid factor.