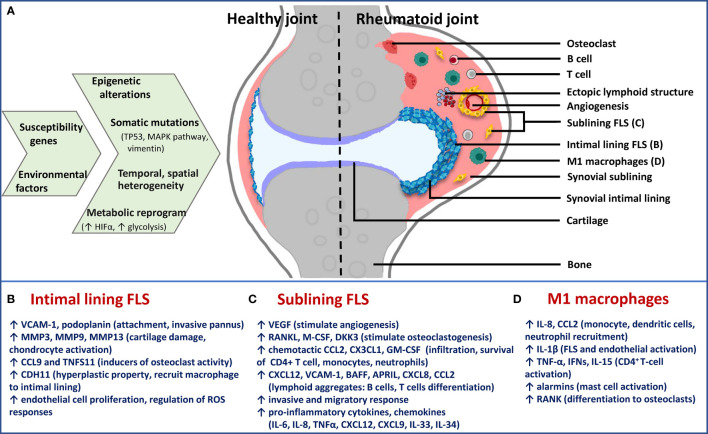
Figure 2.
Schematic presentation of cause-effect relationship in RA synovium with specific focus on FLS phenotypes. (A) Epigenetic and metabolic reprogramming in resident and infiltrated cell types leads to spatiotemporal and functional heterogeneity in rheumatoid synovium. Difference between healthy and rheumatoid joint is diagrammatically depicted to illustrate changes in synovial architecture like increased angiogenesis and formation of ectopic lymphoid structures, as well as the different cell types. Functionally distinct fibroblast subsets: (B) Lining FLS; (C) sublining FLS; as well as (D) inflammatory (M1) macrophages are the major players in chronic RA. For each of them, effector functions via secreted inflammatory and destructive mediators are mentioned below the diagram. Immune-effector fibroblasts and macrophages of sublining are responsible for accelerating inflammatory feed-forward loops, whereas lining fibroblasts mediate bone and cartilage damage. TP53, tumor protein p53; MAPK, mitogen-activated protein kinase; HIFα, hypoxia-inducible factor alpha; FLS, fibroblast-like synoviocytes; M1, inflammatory; VCAM-1, vascular cell adhesion molecule 1; MMP, matrix metalloproteinase; CCL, chemokine (C-C motif) ligand; CDH11, cadherin 11; VEGF, vascular endothelial growth factor; RANKL, receptor activator of nuclear factor kappa-B ligand; M-CSF, macrophage colony-stimulating factor; DKK3, dickkopf WNT signaling pathway inhibitor 3; CXCL, chemokine (C-X-C motif) ligand; GM-CSF, granulocyte-macrophage colony stimulating factor; BAFF, B-cell activating factor; APRIL, a proliferation-inducing ligand; IL, interleukin; TNF-α, tumor necrosis factor alpha.