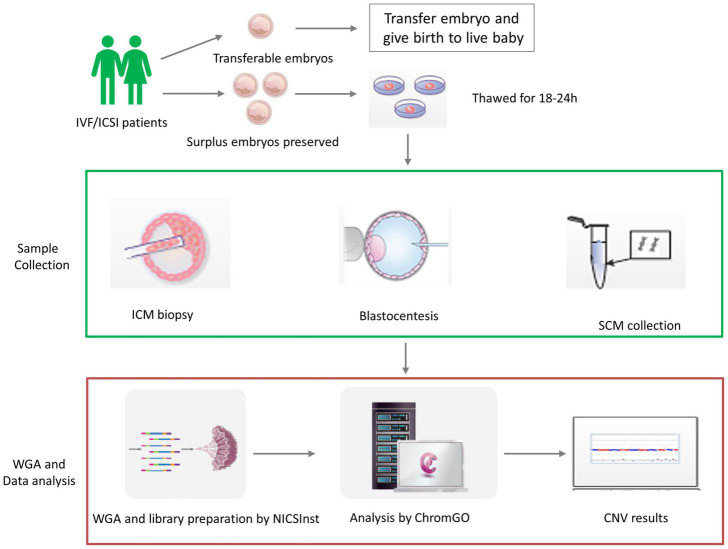
FIGURE 1.
The validation procedure of the study design. Human blastocysts were donated by in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) patients with preserved surplus embryos after giving birth to live healthy babies. We then validated non-invasive chromosome screening (NICS) using spent culture medium (SCM) versus blastocoel fluid (BF) as a source of DNA. In each embryo, we obtained the chromosome ploidy results from the inner cell mass (ICM), trophectoderm biopsy (TE), SCM, and BF after whole-genome amplification (WGA) and library preparation by NICSInst. Copy number variation (CNV) was determined by ChromGo Analysis Software.